Reference: June 2024 | Issue 6 | Vol 10 | Page 62
It is estimated that approximately 7-to-8 per cent of adults have pain with neuropathic characteristics, including a quarter of people with diabetes and 35 per cent of people with HIV have neuropathic pain.
Neuropathic pain, defined by the International Association for the Study of Pain (IASP, 2015) as “pain caused by a lesion or disease of the somatosensory nervous system”, is associated with an impaired quality-of-life and a significant socio-economical impact.
Yet despite the ‘definition’, the management of neuropathic pain can be challenging and, as with all pain, it needs to be approached with a biopsychosocial framework. There are several options for drug and non-drug treatments as part of an overall approach to improve patients’ quality-of-life and function.
This article seeks to give an overview of medication-focused treatment recommendations and highlight future possible targets in this field.
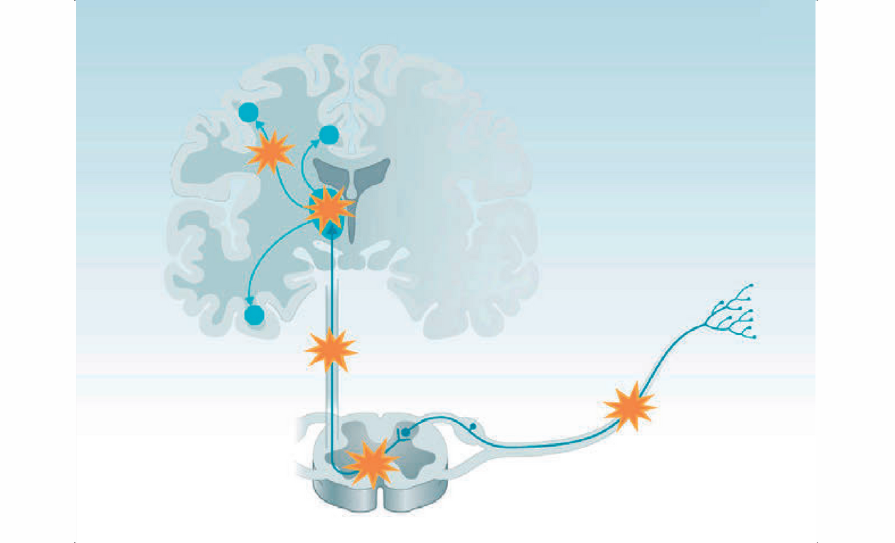
FIGURE 1: Neuropathic pain pathway Source: Finnerup NB, et al. Neuropathic pain: From mechanisms to treatment. Physiol Rev. 2021 Jan 1;101(1):259-301
Mechanism of neuropathic pain
Pain is our natural warning system. When tissue is damaged, specific receptors are activated and an extensive nerve fibre system carry this information from the periphery to the pain centre in the brain. Normally, this is self-limiting so once the tissue heals then the danger is adverted. However, in some cases a ‘lesion’ in the normal pathways can occur, pain persists, and a condition termed ‘neuropathic pain’ evolves.
The concept that pain could be caused by a nerve lesion was first proposed by the medieval medical scholars Rhazes, Haly Abbas, Avicenna, and Jorjani in the 10th and 11th Century. They named this type of pain vaja al asab (nerve-originated pain). Whilst Rhazes described the properties of nerve-originated pain, Haly Abbas described its quality (numbness, tingling, and needle-like).
The first animal model of neuropathic pain was created in 1988 (Bennett and Xie) using a chronic constriction injury of the sciatic nerve in rats. This was followed by a dramatic increase in the development of animal models of neuropathies.
A decade later, in 1998, the first two large-scale multicentre randomised controlled trials (RCTs) of drug therapy (gabapentin) in neuropathic pain (painful diabetic neuropathies and postherpetic neuralgia) were published in The Journal of the American Medical Association.
This marked the beginning of the modern age for neuropathic pain research and it was followed by an exponential increase of pre-clinical and clinical research. For example, the citation rate for neuropathic pain rose from 529 per year in 1998 to more than 3,000 per year since 2019 and 3,256 in 2022. The IASP designated 2014-15 the Global Year against Neuropathic Pain in an effort to highlight the enormous need to identify those who need treatment.
Initially, new discoveries in the mechanisms of persistent pain pathophysiology drove specific areas of research and developments including target-specific therapies. The intricate interplay of multiple cellular and subcellular components alongside the ubiquitous presence of these components on non-nociceptive structures has complicated progress due to the unwanted side-effects. For this reason there have been limited new agents available for commercial use in the last 10 years.
Areas of potential
Four novel areas of pain research show promise in developing both a better understanding of pain responses and new target-specific analgesics for persistent pain. Targeting of persistent pain mechanisms is focused on preventing or altering neural plasticity. Specific receptor or transmitter agents are acting on key steps in the pain cascade.
1) Anti-nerve growth factor antibodies
Nerve growth factor (NGF) is a neurotropin implicated in inflammation and sensitisation phenomenon. Increased concentrations have been found in tissues undergoing an inflammatory response. Biochemically, it is a polypeptide that binds to tropomyosin receptor kinase A (TrKA) and p75 receptors. TrKA receptors on mast cells trigger histamine release, potentiating the inflammatory pathway, and eventual pain hypersensitivity.
By using anti-NGF monoclonal antibodies we may be able to intervene early in histamine activation and affect pain at the transcriptional level by decreasing production of cellular and subcellular elements critical to this pathway.
Three antibody agents have been studied. Tanezumab, a human immunoglobulin-2 NGF antibody, is being studied for osteoarthritis (OA), chronic low back pain, and metastatic bone lesions, among others. It has been found to be highly specific and selective for NGF, and in 2017 it was given fast-track status by the US Food and Drug Administration (FDA).
Meta-analysis of phase 3 studies showed favourable outcomes versus placebo, naproxen, and oxycodone. Fasinumab is in the final stages of trials for hip and knee OA. Fulranumab is a third agent that is no longer undergoing clinical investigation.
Though there was promising initial data to support NGF antagonists as an analgesic modality, the current agents studied generally have side-effects that outweigh the benefits, particularly at higher doses. It is hoped that dosing studies and further trials will elucidate a more favourable profile.
2) Microglia cells
Microglia are cells of the reticuloendothelial system of the CNS. They are responsible for shaping and reshaping neuronal circuits continuously throughout an entire life span. They comprise 10-to-20 per cent of all CNS cells, with the highest densities in the hippocampus, basal ganglia, substantia gelatinosa, and spinal cord. They are responsible for a significant portion of what is termed ‘neuroplasticity’ and microglia cells are involved in the cellular processes that take place during and after nerve injury, one of the pathognomonic features of neuropathic pain.
Microglial activation occurs through several pathways. Perhaps the most important is the mitogen-activated protein kinase (MAPK) pathways. These systems affect intracellular transcription and play a critical role in inflammation. Microglial p38 MAPK pathways are activated via phosphorylation by many proinflammatory cytokines and mediators, including tumour necrosis factor-α (TNF-α), interleukins (such as IL-1β), and adenosine triphosphate (ATP).
It is now possible to influence microglia cells using electrical current. This is the basis of differential target multiplexed spinal cord stimulation (DTM-SCS). It has been studied in the setting of targeting spinal anatomy known to be richer in glial cells, particularly the T8-T11 region. Ongoing human studies targeting these regions with DTM-SCS have produced results showing improved analgesic outcomes over conventional SCS for back and leg pain.
The impact of microglial on neuronal plasticity and the ability to modulate this response may have large implications in the treatment, prevention, and management of chronic pain syndromes. A better understanding and development of pharmaceutical and neuromodulator interventions for these pathways could open a window of opportunity. It may allow physicians to reduce the burden of chronic pain after nerve injury, including post-operative pain syndromes.
3) AMPK activators
AMPK is an enzyme involved in cellular homeostasis that maintains cellular energy levels by regulating ATP production and consumption. When a cellular stressor such as hypoxia, hypoglycaemia, or chemical insult affects a cell, ATP levels decrease, which activates AMPK to restore the balance. AMPK also acts as an inhibitory regulator of several enzymatic pathways linked to chronic pain, including MAPK and mammalian target of rapamycin complex 1.
Mouse model studies have demonstrated that AMPK activation can reduce peripheral nerve injury and allodynia within seven days of injury. AMPK activators have also demonstrated complete resolution of long-standing nerve injury within 60 days of the event. There are rich opportunities for further study and expansion within this field.
4) Genetic studies
Knowledge in this area is still expanding. Genetic studies have revealed that nociception, chronic pain syndromes, and analgesic management all have links to heritable traits. For example, some patients may suffer from painful conditions entirely caused by a genetic mutation resulting in a defective channel protein. Is there a specific mutation that increase the risk of neuropathic pain? Can we genotype families at risk?
There is little use of genetic testing in practice today. Looking to the future, genetic mapping and screening may help provide direction for pharmaceutical management and abuse risk. Gene therapies may represent a means to affect and interact with these complex systems to prevent or treat acute and chronic pain.
Evidence-based treatment
Due to the uncertainty of the pathophysiology, treating neuropathic pain is difficult. New treatments require clinical trials and standards of quality to provide evidence-based recommendations for its pharmacological treatment.
Monotherapy recommendations
The Neuropathic Pain Special Interest Group (NeuPSIG) of the IASP conducted a systematic review of randomised double-blind studies of oral and topical pharmacotherapy for neuropathic pain, including unpublished trials (retrieved from clinicaltrials.gov and pharmaceutical websites). Meta-analysis used numbers needed to treat (NNT) for 50 per cent
pain relief as the primary measure and assessed publication bias. Table 1 highlights the key aspects.
In 229 studies reviewed, the trial outcomes were generally modest even for effective drugs: In particular, NNT were 3.6 [95% CI 3.0-4.4] for tricyclic antidepressants (TCAs), 6.4 [95% CI 5.2-8.4] for serotonin-noradrenaline reuptake inhibitor (SNRI) antidepressants duloxetine and venlafaxine, 7.7 [95% CI 6.5-9.4] for pregabalin, and 6.3 [95% CI 5.0-8.3] for gabapentin. NNT were higher for gabapentin ER and capsaicin high concentration patches, lower for opioids and botulinum toxin A (BTX-A), and undetermined for lidocaine patches. Final quality of evidence was lower for lidocaine patches and BTX-A.
Limited efficacy, large placebo responses, inadequate diagnostic criteria, and poor phenotypic profiling probably account for modest trial outcomes and should be taken into account in future studies. A number of overarching themes were identified in the research:
a) Most studies were conducted in diabetic neuropathy or postherpetic neuralgia;
b) Publication bias accounted for approximately 10 per cent of the
treatment effect;
c) Placebo effect was large;
d) Drug effects were modest;
e) Data did not identify that one particular drug or drug class was superior in any particular neuropathic pain syndrome;
f) The majority of studies were for 12 weeks or less;
g) Data were limited to non-cancer pain in adults.
Neuropathic agents head-to-head
To help understand how agents preformed within and across different products, Sadegh et al (2024) conducted a systematic review and meta-analysis of direct-comparison double-blind randomised trials comparing neuropathic agents.
Primary outcomes included mean change in pain intensity and the number of responders with a 50 per cent reduction in pain intensity. Secondary outcomes encompassed quality-of-life, sleep, emotional functioning, and number of dropouts because of adverse events. They included 30 trials (4,087 patients), comprising 16 crossover and 14 parallel groups design studies. All studies were conducted in adults and the majority were investigator-initiated trials.
a) They found moderate-quality evidence for equivalence (no clinically relevant difference) between TCA and gabapentin/pregabalin with a combined mean difference in pain score of 0.10 [95% CI 20.13-0.32].
b) There was no documented differences between TCA and SNRI, between SNRI and gabapentin/pregabalin, or between opioids and TCA (low quality of evidence).
c) The dropouts rate in studies was because of adverse events with SNRI and opioids compared with TCA (low quality of evidence).
d) They did not identify any studies that included topical treatments. This systematic review of direct comparison studies found evidence for equivalence between TCA and gabapentin/pregabalin and fewer dropouts with TCA than SNRI and opioids.
| CLASSIFICATION | AGENT | MECHANISM OF ACTION | TYPICAL DOSE RANGE | NUMBER NEEDED TO TREAT | NUMBER NEEDED TO HARM |
|---|---|---|---|---|---|
| FIRST-LINE | |||||
| Tricyclic antidepressants | Amitriptyline Nortriptyline | Monoamine reuptake inhibition, Sodium channel blockade and anticholinergic effects | 10-150mg/day | 3.6 [95% CI 3.0-4.4] | 13.4 (9.3-24.4) |
| SNRI | Duloxetine Venlafaxine | Serotonin and noradrenaline reuptake inhibitors | 30-120mg daily 150-225mg daily | 6.4 [95% CI 5.2-8.4] | 11.8 (9.5-15.2) |
| Gabapentinoids | Pregabalin Gabapentin | Alpha-2-delta calcium block | 150-600mg/day 900-3,600mg/day | 7.7 [95% CI 6.5-9.4] 6.3 [95% CI 5.0-8.3] |
13.9 (11.6-17.4) 25.6 (15.3-78.6) |
| SECOND-LINE | |||||
| Tramadol, Lignocaine 5 per cent, Capsaicin 8 per cent | Sodium channel block TRPV1 receptor | 400mg daily Apply 12 hours on/off Apply once every 90 days |
6.4 | 2.6 (8.4-25.3) | |
| THIRD-LINE | |||||
| Opioids | Tramadol ER Oxycodone Morphine | Opioid Mu-receptor | 400mg daily 10-120mg/day 90-240mg/day |
6.4 4.3 (3.4-5.8) 4.3 (3.4-5.8) |
2.6 (8.4-25.3) 1.7 (8.4-19.3) 1.7 (3.4-5.8) |
TABLE 1: Overview of common neuropathic agents, mechanism of action, typical dose range, number needed to treat and number needed to harm
Modified from several sources
Pragmatic management
Clearly neuropathic pain is a challenging chronic pain condition. Due to limited evidence-based knowledge, the relative effectiveness of pharmacological treatments, and differences in trial design, and impact of the placebo response preclude indirect comparisons of efficacy between drug classes. Prescribers need to consider a number of factors when they consider starting on medication.
a) Use a recognised screening tool to establish the true existence of neuropathic pain. For example, the Douleur Neuropathique 4 questionnaire (DN4) or PainDetect scores are easy bedside options. Monitoring for efficacy (using multidimensional tools for pain intensity, quality-of-life, and patient function) and safety can provide valuable insight.
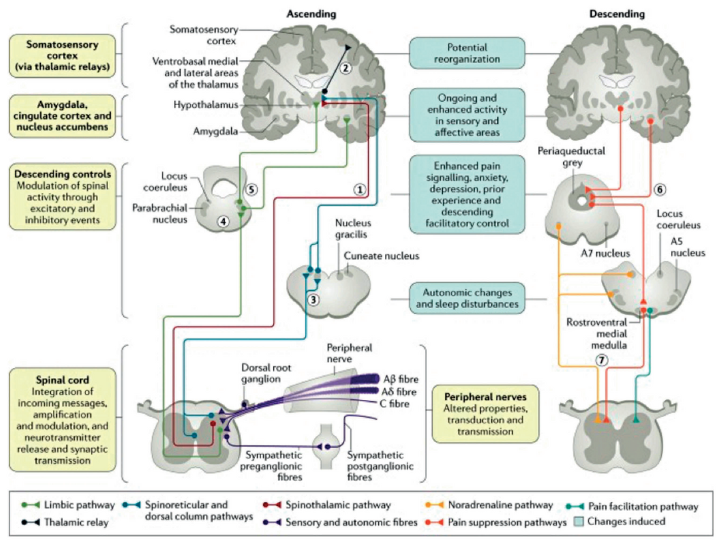
FIGURE 2: Neuropathic pain pathway Source: Colloca L, et al. Neuropathic pain. Nat Rev Dis Primers. 2017 Feb 16;3:17002
b) Consider Quantitative Sensory Testing to assist in making this diagnosis and potentially guide treatments.
c) Use the IASP guidelines to choose the agents. Start with a TCA or SNRI with consideration of the patient’s comorbidities, potential drug interactions and adverse effects, and consider pregabalin or gabapentin next before tramadol. The gabapentinoids have neurocognitive adverse effects, can cause weight gain, and are associated with an increased risk of falls. They are anxiolytic, and there is emerging evidence of significant pregabalin abuse.
d) Set the duration of the treatment
and identify outcomes you see as valuable to the patient. A pragmatic approach may be to try a therapy for 12 weeks as this is the maximum duration of most of the trials. Continue to monitor for efficacy (using multidimensional tools for pain intensity, quality-of-life, and patient function) and safety.
e) Finally, consider the dose regime, stop if the treatment is not working, and agree the plan with the patient from the beginning.
In summary, neuropathic pain is one of the most incapacitating pains and represents a significant unmet medical need. It is estimated that approximately 7-to-8 per cent of adults have pain with neuropathic characteristics. We need to use the pharmacological options wisely to ensure the best and safest treatment can be provided to patients.
References on request