HIQA has published its health technology assessment (HTA) of shingles (herpes zoster) vaccination for adults, following a public consultation that took place earlier this year.
The Authority undertook this assessment at the request of the Department of Health following a clinical recommendation from the national immunisation advisory committee (NIAC). This HTA has been submitted as advice to the Minister for Health to inform a policy decision on whether to provide shingles vaccination as part of the adult immunisation programme in Ireland.
Dr Conor Teljeur, HIQA’s Chief Scientist, said: “We are grateful to all who participated in the public consultation. The input we received was carefully considered in this final assessment, and much of it is reflected in sections regarding burden of the disease, patient aspects and social aspects.
“Our assessment concludes that the shingles vaccine is safe and effective, but that the benefit of the vaccine decreases over time. We found that, at the current vaccine price, adding shingles vaccination to the routine immunisation schedule for the general population aged 50 years and older would not be an efficient use of HSE resources.”
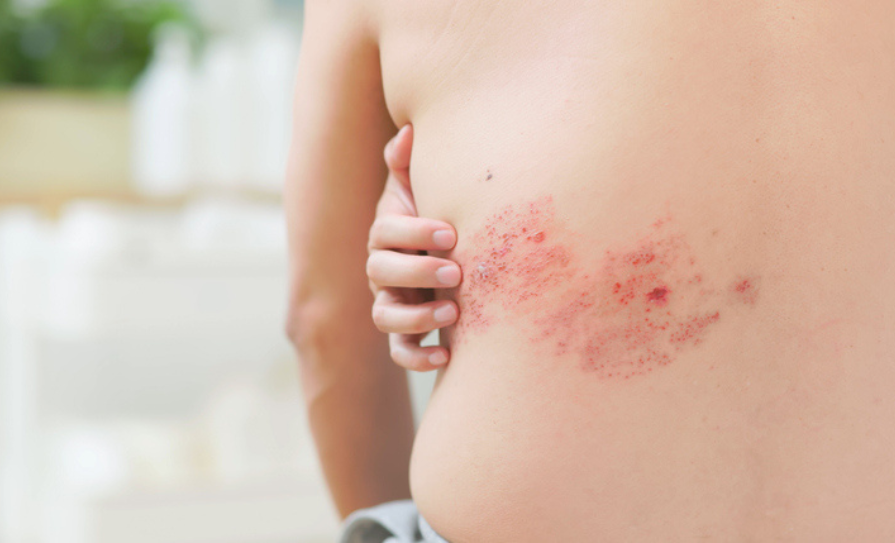
Shingles is a viral infection caused by the same virus that causes chickenpox. Shingles causes a painful, blister-like rash. While some people experience severe disease or continue to experience pain for months, or even years, for most people symptoms normally resolve within a month.
Feedback from the public consultation highlighted the significant impact that shingles can have on individuals who experience longer-term complications. While shingles vaccines are available in Ireland, the HSE does not currently provide free vaccination — people must pay to be vaccinated.
The incidence and severity of shingles increases with age, with most cases occurring in people over the age of 50 years. People who are immunocompromised are also at increased risk of shingles and experiencing a severe disease course. HIQA assessed the impact of providing shingles vaccination for adults aged 50 years and older, and for those aged 18 years and older who are at increased risk due an immunocompromising condition or treatment.
The HTA is available here – https://www.hiqa.ie/sites/default/files/2024-07/HZ-vaccination-HTA.pdf