Diabetes mellitus is the leading cause of chronic kidney disease (CKD) and end stage renal disease (ESRD) globally. Around 20-40 per cent of diabetics develop diabetic kidney disease (DKD). This is a clinical syndrome characterised by progressive decline in estimated glomerular filtration rate (eGFR); elevated arterial blood pressure (BP); and persistent albuminuria (>300mg/d) on at least two visits, three to six months apart.
Structural changes include thickening of the glomerular basement membrane (GBM), mesangial expansion, podocyte injury, and glomerulosclerosis. Extraglomerular lesions are also involved in the progression of the disease, including tubular atrophy, interstitial inflammation, and tubulointerstitial fibrosis.
Functional changes encompass a paradoxically high eGFR in the early stages of the disease, termed glomerular hyperfiltration, caused by afferent arteriolar vasodilation and/or by efferent arteriolar vasoconstriction owing to activation of the renin-angiotensin-aldosterone system (RAS), leading to glomerular hypertension. Later, proteinuria, systemic hypertension, and loss of renal function develop.
Risk factors for DN
Non-modifiable risk factors for DN include increasing age, family history, and genetic factors, with genes such as ACE, APOC1, GREM1, UNC13B, ALR2, APOE associated with the disease. DN is also more common in black people, Mexican Americans, Pima Indians, and Hispanics compared to Caucasians, and female gender is associated with a reduced risk of progression from moderate albuminuria to severe albuminuria or ESRD.
Modifiable risk factors include smoking; longer durations of diabetes; obesity; hypertension; poor glycaemic control; and dyslipidaemia (abnormal lipoprotein metabolism is accelerated in DN that causes further renal injury, leading to ESRD).
Clinical features
The most common clinical abnormalities of DKD are persistently elevated urine albumin excretion and/or persistently declining eGFR. These manifestations tend to be asymptomatic, being detected through routine periodic testing. For this reason, type 2 diabetics should undergo testing at the time of diagnosis, and yearly thereafter.
On some occasions, patients can complain of fatigue, foamy urine, and pedal oedema due to hypoalbuminaemia and nephrotic syndrome. They may also have associated peripheral vascular disease, hypertension, cardiovascular disease (CVD), and diabetic retinopathy.
Investigations
1. Routine blood tests: Renal profile, full blood count, electrolytes.
2. Urine albumin excretion: Albuminuria can be established if two to three urine collections obtained over three to six months show elevated levels of albumin.
3. Urine culture to exclude infection and microscopy to examine for red cell casts in glomerulonephritis.
4. Anti-DNA antibodies, antinuclear antibody, extractable nuclear antigen, complement levels, anti-neutrophil cytoplasmic antibodies, antistreptolysin O titre, rheumatoid factor, and anti-glomerular membrane antibody to check for autoimmune disease.
5. Serum protein electrophoresis, immunoglobulins, urine protein electrophoresis for multiple myeloma.
6. Renal ultrasound.
7. Renal biopsy: The gold standard, but rarely used.
| CLASS | DESCRIPTION AND CRITERIA |
|---|---|
| I | GBM thickening on electron microscopy: GBM >395nm (female), GBM >430nm (male) |
| IIa | Mild mesangial expansion |
| IIb | Severe mesangial expansion. A lesion is considered severe if areas of expansion larger than the mean area of a capillary lumen are present in >25 per cent of total mesangium |
| III | At least one Kimmelstiel-Wilson lesion (nodular intercapillary glomerulosclerosis) and there is <50 per cent global glomerulosclerosis |
| IV | Advanced diabetic glomerulosclerosis. There is >50 per cent global glomerulosclerosis, attributable to diabetic nephropathy |
TABLE 1: Tervaert classification
Classification
Tervaert classification provides a systematic approach with regards to the classification of the pathology of DN and gives a guide with regards to the prognosis of the disease. An important limitation of this classification scheme is that not all pathologic lesions are included, such as presence of mesangiolysis, capillary aneurysms, exudative lesions, and focal/segmental sclerosis.
Treatment
There is no definitive cure for DKD, with management focused on lifestyle interventions and optimal glucose and BP control.
Lifestyle interventions
Input from a dietitian is often indicated for this patient cohort. Patients with diabetes and CKD should generally consume a diet rich in vegetables, fruits, fibre, legumes, plant-based proteins, unsaturated fats, and nuts whilst avoiding processed meats, sweetened beverages, and refined carbohydrates. In advanced CKD, however, potassium in particular, needs to be restricted.
Nutrition therapy can reduce levels of Hba1c to similar or even better ones to those achieved with glucose-lowering medications. However, too much protein can lead to reduced carbohydrate intake with consequent weight loss, and such diets can cause harm to kidney function due to increased urinary excretion of amino acids, which can elevate acid load and precipitate metabolic acidosis, especially in patients with poor kidney function.
On the other hand, in very limited studies, protein restriction has been associated with a slower decline in eGFR in non-diabetics with CKD. Most type 2 diabetic CKD patients would have already been counselled on the appropriate carbohydrate and fat intake, and with protein restriction, malnutrition, reduced quality of life, and hypoglycaemia can develop.
In view of the lack of clinical trials, guidance for such patients is based on the World Health Organisation recommendations for protein intake of 0.8g/kg/day being associated with good outcomes. Patients on dialysis are recommended to consume 1.0-to-1.2g/kg/day as dialysis causes a catabolic response, with loss of amino acids. In addition, the presence of uraemia promotes decreased appetite, increased catabolism, and reduced muscle mass.
Low sodium intake is associated with lower BP and improved cardiovascular (CV) outcomes in the general population. Patients with CKD tend to be salt-sensitive and unable to regulate BP and extracellular fluid volume status when consuming high salt diets. Low salt intake is associated with improvement in volume status and reduced proteinuria, while high sodium intake is associated with increased mortality and morbidity. The guidelines advise that sodium intake should be restricted to <2g/day or <90mmol of sodium/day (<5g of sodium chloride/day).
Engaging in physical activity offers cardiometabolic, kidney, and cognitive benefits. Weight loss may reduce urinary albumin excretion and improve BP. Physical activity lowers inflammatory markers, improves insulin sensitivity and endothelial function, and is associated with slower decline in eGFR.
Therefore, it is recommended that CKD patients with diabetes perform at least 150 minutes of moderately intense physical activity per week. Nonetheless, such patients tend to be elderly with increased risk of falls, obese, and anaemic, with further limitations in their functional capacity, so care needs to be taken.
Use of tobacco is a leading cause of death and also promotes the development of CKD, with a higher incidence of CV events noted among current and former smokers in diabetics with CKD versus never smokers. Second hand exposure is also associated with CKD and kidney disease.
Therefore, diabetics with CKD, along with the general population, are advised to stop using tobacco products, with the help of pharmacotherapy and behavioural support. E-cigarettes are not recommended due to emerging links with lung cancer and CVD, and their impact on kidney disease is not fully known.
Pharmacological therapy
Angiotensin-converting enzyme inhibitor (ACEi) or an angiotensin II receptor blocker (ARB): It is recommended that patients with diabetes, hypertension, and albuminuria are started on ACEi or ARB, with dose titration as needed. Albuminuria is associated with increased risk of progression of CKD, and ultimately kidney failure, and with increased risk of CVD. Several trials have shown that through RAS (renin-angiotensin-system) blockade, ACEi, and ARBs were effective in reducing albuminuria and even reversal of moderately increased albuminuria, slowing the rate of kidney function loss.
Use of ACEi/ARBs in type 2 diabetes (T2D), albuminuria, and without hypertension are beneficial. However, their use is not beneficial for patients with neither albuminuria nor elevated BP. For CKD patients with T2D and hypertension, but normal urine albumin excretion, BP control with any anti-hypertensive class is important to prevent CVD.
Serum creatinine, eGFR, and potassium should be measured within two to four weeks of starting treatment or making a change in the dose of ACEi/ARBs. These drugs block the action of angiotensin II, leading to selective vasodilatation of the efferent arterioles, resulting in a decrease in the intraglomerular pressure with consequent rise in creatinine and decrease in eGFR, and block the action of aldosterone, potentially leading to hyperkalaemia. ACEi should not be combined with ARBs as this can lead to hyperkalaemia and acute kidney injury (AKI).
It is also important to advise contraception for women of child-bearing age or to discontinue treatment in women who are pregnant or planning to conceive, as ACEi/ARBs are associated with neonatal complications, especially when continued in the second/third trimester including:
- Impaired foetal or neonatal kidney function resulting in oligohydramnios during pregnancy and kidney failure after delivery;
- Pulmonary hypoplasia;
- Limb defects;
- Cerebral complications;
- Miscarriages or perinatal death.
ACEi can also cause dry cough and angioedema due to inhibition of bradykinin. Switching to ARBs is an option in such cases. Treatment should begin with the lowest possible dose and titrate to the maximum tolerated one, as with increasing dose, side-effect risks increase.
Sodium-glucose cotransporter-2 inhibitors (SGLTi): SGLTi are recommended for the treatment of patients with T2D, CKD, and eGFR ≥20ml/min per 1.73m2. These drugs inhibit kidney tubular reabsorption of glucose leading to lower blood glucose. In view of this glycosuria, a diuretic effect is caused, leading to increased urine output. SGLTi also alter metabolism by shifting away from carbohydrate utilisation to ketogenesis, leading to lower HbA1c, BP, and weight. In addition, they lead to a reduction in intraglomerular pressure and subsequent preservation of kidney function.
SGLTi are associated with CV and heart failure (HF) benefits, with reduction in CV death, all-cause mortality, and HF hospitalisation compared to placebo. Kidney benefits include a slower decline in eGFR, reduction in albuminuria, and reduced risk of dialysis, kidney transplant, and death from renal causes.
With regards to initial therapy for patients not yet started on glucose-lowering drugs, different guidelines recommend different regimens, with some suggesting starting with metformin and others starting with SGLTi. Based on the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines, it is recommended that most T2D CKD patients with an eGFR ≥30ml/min per 1.73m2:
- Start with a combination of metformin and SGLT2i;
- Once eGFR declines to 30-45ml/min/1.73m2, maximum dose of metformin should be halved;
- Once eGFR <30ml/min/1.73m2 or the patient is started on dialysis, metformin is stopped;
- Metformin can be administered to kidney transplant patients as long as eGFR is ≥30ml/min per 1.73m2 ;
- Once eGFR <20ml/min/1.73m2 one can continue treatment with SGLTi;
- Very little data is available once patients are started on dialysis or have received a kidney transplant, so discontinuation of SGLTi is recommended at this stage;
- SGLTi should not be started if eGFR is ≤20ml/min per 1.73m2.
When used as monotherapy, the risk of hypoglycaemia with SGLTi is low as the drug-induced glycosuria decreases as blood glucose normalises. However, when used with other therapies that can cause hypoglycaemia, the dose of these pre-existing medications needs to be adjusted. Withhold SGLTi during times of prolonged fasting or surgery, when patients may be at a greater risk of ketosis.
In patients at risk of hypovolaemia, reduce thiazide or loop diuretic before starting treatment with SGLTi, as SGLTi are associated with an initial natriuresis. SGLTi can cause a reversible decrease in eGFR. An eGFR drop of ≤30 per cent should be tolerated and should not lead to discontinuation of treatment. If eGFR drops further, ensure the patient is not hypovolaemic, adjust diuretic dose, and seek other possible causes of the AKI. CKD or HF patients without T2D can be started on SGLTi, as they offer CV and kidney protection without conferring an increased risk of hypoglycaemia or diabetic ketoacidosis.
Diabetic ketoacidosis is a rare side-effect of SGLTi, as is an increased risk of genital mycotic infections.
Nonsteroidal mineralocorticoid receptor antagonist: Nonsteroidal mineralocorticoid receptor antagonist (MRA) are recommended for T2D patients with ≥eGFR 25ml/min/1.73m2, normal serum potassium concentration, and albuminuria ≥30mg/g despite on maximum tolerated dose of ACEi/ARBs. Through the use of ACEi/ARBs, there is kidney and CV benefit via RAS blockade.
Nonetheless, data has shown that there is incomplete suppression of serum aldosterone levels, thereby suggesting the need for further treatment to reduce residual albuminuria. Steroidal MRA are used to treat primary hyperaldosteronism levels, HF, and to reduce albuminuria, but data is lacking with regards to their effect on kidney disease progression. Moreover, they can cause hyperkalaemia and AKI, and spironolactone is associated with gynaecomastia.
Novel nonsteroidal MRA such as finerenone are more selective for mineralocorticoid receptors, and confer CV and renal benefits with reduced albuminuria and slower decline in eGFR. They are also associated with a lower risk of hyperkalaemia compared to steroidal MRA.
Patients with T2D, CKD, and albuminuria on SGLTi and ACEi/ARBs can also start taking finerenone, provided that they have a normal serum potassium and albumin to creatinine ratio is ≥30mg/g. Use of SGLTi also reduces the risk of hyperkalaemia in patients already on ACEi/ARBs and finerenone. Moreover, finerenone can be added to patients on ACEi/ARBs only, despite not being on SGLTi.
A steroidal MRA is used in the treatment of HF, hyperaldosteronism, and refractory hypertension. Clinical evidence is lacking whether switching from a steroidal to nonsteroidal is associated with an improvement in clinical outcome. When the patient is treated with neither and has T2D, HF, and albuminuria and is already on ACEi/ARBs and SGLTi, treatment should be based on the most concerning clinical indication. At present, a nonsteroidal MRA cannot replace a steroidal MRA for HF and hyperaldosteronism.
Finerenone can cause hyperkalaemia, and monitoring of this electrolyte is important. Treatment with finerenone should not be started if serum potassium is >5mmol/l. Finerenone has a short half-life, therefore stopping the drug for 72 hours will lead to resolution of the elevated potassium.
Steroidal and nonsteroidal MRA should not be combined due to risk of hyperkalaemia. Steroidal MRA are currently contraindicated in pregnancy, and in view of the lack of clinical data of the use of nonsteroidal MRA in pregnancy, this drug should be stopped.
Other anti-hypertensive treatment: KDIGO guidelines recommend that patients with albuminuria, T2D, CKD, and hypertension are started on ACEi/ARBs until maximum tolerated dose. If patients have normal serum potassium and albumin to creatinine ratio is ≥30mg/g, finerenone can be added. If this is not the case and BP is still high, dihydropyridine calcium channel blocker and/or diuretic can be added. Should BP still remain high and eGFR ≥45, steroidal MRA can be added.
Glucagon-like peptide-1 receptor agonists: In T2D CKD patients who have not achieved the desirable glycaemic targets despite lifestyle interventions, treatment with metformin and SGLT2i, or in whom the latter two medications are not tolerated, guidelines recommend prioritising glucagon-like peptide-1 receptor agonists(GLP-1 RA) over other glucose lowering therapies.
GLP-1 RA have been shown to improve glycaemic control, confer weight loss and CV benefit, reduce albuminuria, and slow the rate of eGFR decline. In view of their proven CV benefit, GLP-RA are preferred over other glucose-lowering therapies (Dipeptidyl peptidase-4 inhibitor (DPP-4) inhibitors, thiazolidinediones, sulfonylureas, insulin). The risk of hypoglycaemia is generally low when GLP-1 RA are used alone. When used with other medications, the risk is increased, so the dose of sulfonylurea and/or insulin may need to be reduced.
GLP-1 RA may be preferentially used in patients with obesity, T2D, and CKD to promote intentional weight loss. Further studies are required regarding the use of GLP-1RA in patients with very advanced CKD, patients on dialysis, and in kidney transplant recipients. Side-effects of GLP-1 RA include nausea/vomiting, diarrhoea, and increased heart rate. Also, since most of them are given as a subcutaneous injection, they are associated with pain over the injection site. These medications are contraindicated in patients with a history of medullary thyroid carcinoma, multiple endocrine neoplasia 2, and patients with a history of acute pancreatitis.
Other glycaemic treatment: If the glycaemic target is still not achieved despite lifestyle interventions, metformin, SGLTi, and GLP-1 RA, then other glucose-lowering agents can started, such as DPP-4 inhibitors, thiazolidinediones, sulfonylureas, and insulin.
Monitoring of blood glucose in T2D CKD patients
HbA1c is recommended to monitor glycaemic control in these patients, measured twice a year, or up to four times/year should glycaemic targets not be met. However, inflammation, oxidative stress, and metabolic acidosis associated with CKD, as well as anaemia, transfusions, and use of iron replacement therapies and erythropoiesis-stimulating agents, affect HbA1c level. These effects become more pronounced as the CKD advances or patients are treated with dialysis. Once HbA1c levels are not concordant with blood glucose levels, continuous glucose monitoring and self-monitoring of blood glucose can be used, as these are not influenced by CKD, dialysis, or other treatments. One should aim for target HbA1c of <6.5-to-8.0 per cent, with levels ≤6.0 per cent associated with greater risk of hypoglycaemia and increased mortality.
Dyslipidemia treatment
Managing dyslipidaemia, according to KDIGO recommendations, includes:
- CKD patients are at increased risk of adverse events when statins and fibrates are combined. Since statins confer a greater clinical benefit compared to fibrates, statins are preferred.
- Statin or statin/ezetimibe combination in adults aged ≥50 with eGFR ≤60ml/min/1.72m2, not on dialysis and without kidney transplant. Ezetimibe monotherapy is not recommended.
- Statin in adults aged ≥50 with eGFR ≥60ml/min/1.72m2.
- Statin in adults aged 18 to 49 with CKD, but not on dialysis and without kidney transplant if they have coronary disease (myocardial infarction or coronary revascularisation); diabetes mellitus; prior ischaemic stroke; estimated 10-year incidence of coronary death or non-fatal myocardial infarction >10 per cent.
- If at the time of dialysis initiation, patients are already on statin or statin/ezetimibe combination, this can be continued.
- Do not start statin or statin/ezetimibe combination in dialysis patients.
- In adult kidney transplant recipients, treatment with a statin is recommended.
- Statins are contraindicated in pregnant or breastfeeding females, patients with active liver disease or in patients in whom transaminase levels are three times or more the upper limit of normal.
For additional risk-based therapy, aspirin can be used lifelong for secondary prevention among those with established CVD. Ezetimibe or proprotein convertase subtilisin-kexin type 9 inhibitors (PCSK9i) can be added to a statin. PCSK9i can be used in statin-intolerant patients, with these medications showing an improvement in lipid profile and CV risk. Nonetheless, further studies are needed to ascertain their safety in patients with eGFR ≤20ml/min/1.73m2, those on dialysis, and/or renal transplant patients.
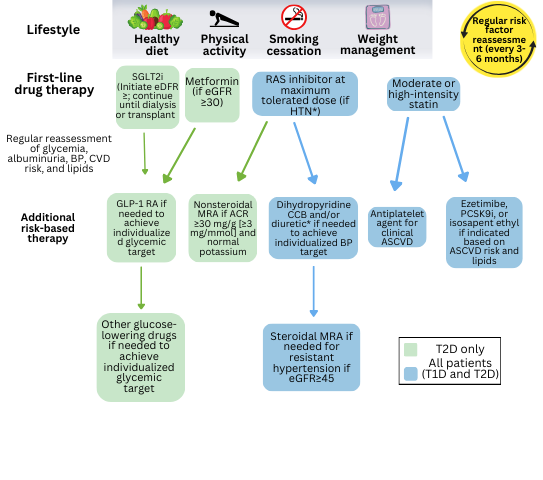
FIGURE 1: KDIGO management of patients with DKD
Conclusion
DKD is a constantly evolving subject with new guidelines issued very frequently. Nonetheless, a patient-centred approach with involvement of relatives and multidisciplinary team is required to achieve the best care possible, including involvement of nutritionist, physiotherapist, personal trainer, smoking cessation support, endocrinologist, cardiologist, GP, and various other medical disciplines.
KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease provides evidence-based recommendations and can be accessed at: www.kdigo.org/wp-content/uploads/2022/10/KDIGO-2022-Clinical-Practice-Guideline-for-Diabetes-Management-in-CKD.pdf.
References available on request