

Resected stage IV melanoma can occur and is high-risk but is not discussed in this material.
What Is High-Risk Resected Melanoma?1,2
Resected stage IV melanoma can occur and is high risk but is not discussed in this infographic.
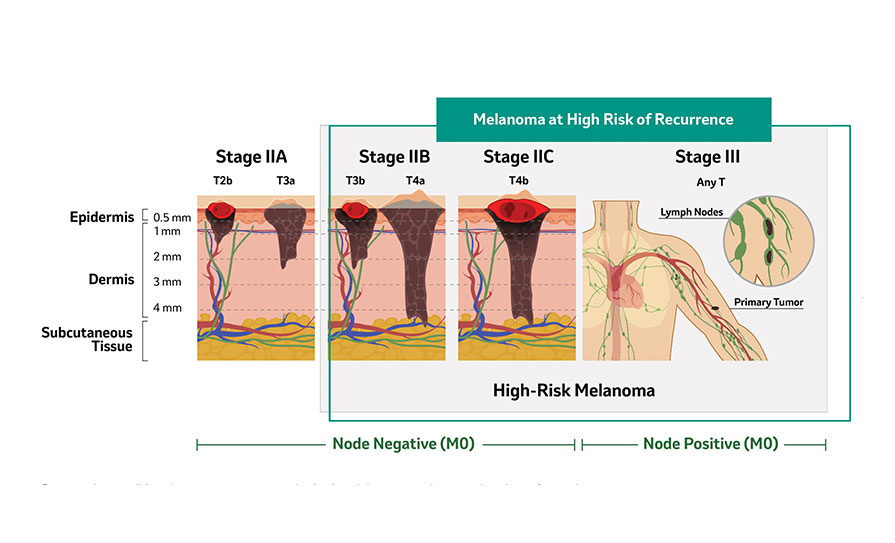
T3a: >2.0–4.0 mm without ulceration; T2b: >1.0–2.0 mm with ulceration; T4a: >4.0 mm without ulceration; T3b: >2.0–4.0 mm with ulceration; T4b: >4.0 mm with ulceration; T: primary tumor; AJCC: American Joint Committee on Cancer; M0: no metastasis
Recurrence Rates for High-Risk Resected Melanoma3,4
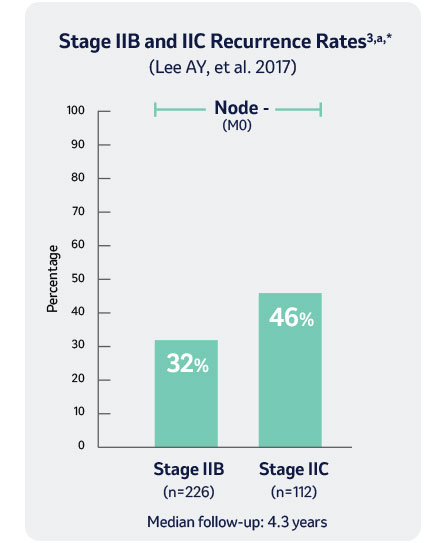
* Retrospective review of 738 adult patients from 1993-2013 with stage II resected melanoma. Patients in this study were treated at Memorial Sloan Kettering Cancer Center, New York, NY, North America.
M0: no metastasis, a According to AJCC 7th Edition Staging
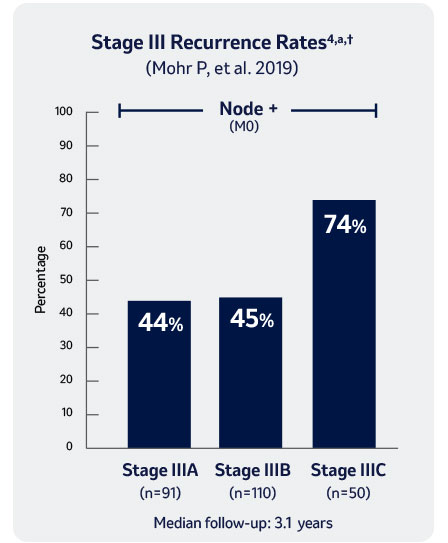
† Retrospective chart review of 251 patients from 2011-2016 with stage III resected melanoma followed by watch-and-wait. Patients included in this study were from North America, South America, and Europe.
Median Time to Recurrence in High-Risk Resected Melanoma3,4
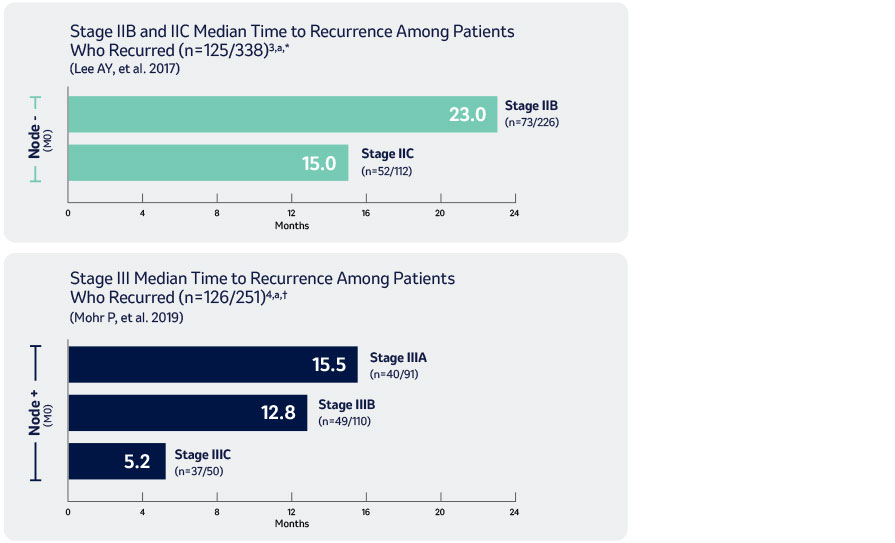
M0: no metastasis
a According to AJCC 7th Edition Staging
* Retrospective review of 738 adult patients from 1993-2013 with stage II resected melanoma. Patients in this study were treated at Memorial Sloan Kettering Cancer Center, New York, NY, North America.
† Retrospective chart review of 251 patients from 2011-2016 with stage III resected melanoma followed by watch-and-wait. Patients included in this study were from North America, South America, and Europe.
Patients Often Recurred With Distant Metastases 3,4
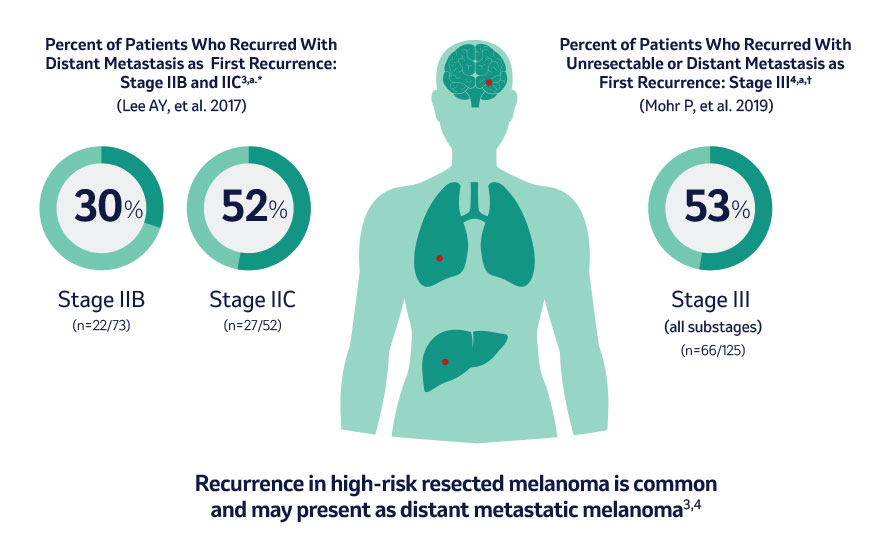
a According to AJCC 7th Edition Staging
* Retrospective review of 738 adult patients from 1993-2013 with stage II resected melanoma. Patients in this study were treated at Memorial Sloan Kettering Cancer Center, New York, NY, North America.
† Retrospective chart review of 251 patients from 2011-2016 with stage III resected melanoma followed by watch-and-wait. Patients included in this study were from North America, South America, and Europe.
References:
- 1. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma of the skin. In: AJCC Cancer Staging Manual. 8th ed. American Joint Committee on Cancer; 2017:563-588.
- 2. Helvind NM, et al. Stage-Specific Risk of Recurrence and Death From Melanoma in Denmark, 2008-2021. JAMA Derm. 2023. doi:10.1001/jamadermatol. 2023.3256
- 3. Lee AY, Droppelmann N, Panageas KS, et al. Patterns and timing of initial relapse in pathologic stage II melanoma patients. Ann Surg Oncol. 2017;24(4):939-946. doi:10.1245/s10434-016-5642-0
- 4. Mohr P, Kiecker F, Soriano V, et al. Adjuvant therapy versus watch-and-wait post surgery for stage III melanoma: a multicountry retrospective chart review. Melanoma Manag. 2019;6(4):MMT33. doi:10.2217/mmt-2019-0015
Category: POM Marketing Authorisation numbers: EU/1/15/1024/002 Marketing Authorisation holder: Merck Sharp & Dohme B.V., Waarderweg 39, 2031 BN Haarlem, The Netherlands.
Date of revision: March 2024.
IE-KEY-00918
© 2024 Merck & Co., Inc., Rahway, NJ, USA and its affiliates.
All rights reserved. Further information is available on request from: MSD, Red Oak North, South County Business Park, Leopardstown, Dublin D18 X5K7 or from www.medicines.ie.
Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie. Adverse events should also be reported to MSD (Tel: 01-299 8700)
Please click here to view the KEYTRUDA SPC on medicines.ie
Before prescribing please read the Summary of Product Characteristics for KEYTRUDA available on medicines.ie





Leave a Reply
You must be logged in to post a comment.