Reference: Nov-Dec 2024 | Issue 6 | Volume 17 | Page 15
Chronic obstructive pulmonary disease (COPD) is a heterogenous lung condition characterised by respiratory symptoms (dyspnoea, cough, sputum production) caused by abnormalities of the airways (bronchitis, bronchiolitis) and alveoli (emphysema) that cause persistent and often progressive airflow obstruction.¹
The condition is common, preventable, and usually caused by significant exposure to noxious particles or gases. The chronic airflow limitation that is characteristic of COPD is caused by a mixture of small airways disease (eg, obstructive bronchiolitis) and parenchymal destruction (eg, emphysema), and can vary from person to person.
General practice nurses have become instrumental in managing these patients as care continues to move away from the acute sector and closer to patients’ homes.
Prevalence
In Ireland, COPD places a significant burden on the healthcare system and adult population. It is estimated that 500,000 people are living with the disease, yet only 200,000 are formally diagnosed.² As set out in the third National Healthcare Quality Report System (NHQRS) 2018, Ireland has the highest rate of hospital admission with COPD of any country in the Organisation for Economic Co-operation and Development (OECD).³ There were 15,127 inpatient hospitalisations with a primary diagnosis of COPD in 2017, accounting for 3.9 per cent of inpatient discharges.³
Globally and nationally, COPD is more prevalent among the vulnerable sections of society, including people from areas with high social deprivation.⁴ In 2019, the National Institute for Health Research in the UK carried out a systematic review which explored the prevalence of COPD from 1990-2019 in 65 countries. The study found that the global prevalence of COPD was 10.3 per cent in 30-79 year olds; equivalent to 391.9 million people.⁴
Risk factors
The risk factors for developing COPD include being male; current smoker; having a body mass index (BMI) of less than 18.5; exposure to biomass fuels; and occupational exposure to dust or smoke.⁴ Other contributing factors include genetic factors, lung growth and development, socioeconomic status, asthma and airway hyper-reactivity, chronic bronchitis, and infections.
In a recent study of 6,098 non-obstructed smokers, 1,781 (29 per cent) developed COPD after five years of follow up. A total of 23 per cent of participants had a <10 pack-years of smoking at baseline, 26 per cent had 10 to 19.9 pack-years, 30 per cent had 20 to 39.9 pack-years, and 39 per cent had ≥40 pack-years.
The paradigm of COPD as a predominantly male disease is changing as smoking rates in both males and females are now similar and the number of deaths due to COPD among women has surpassed that of men. Women appear more susceptible to the effects of cigarette smoke, developing COPD earlier and with lower cigarette exposure than men. Women also commonly exhibit a COPD phenotype with airway dominant disease in comparison with emphysema, and they also vary in response to treatment.
| IN PATIENTS WITH FEV₁/FVC <70 PER CENT: | ||
|---|---|---|
| GOLD | Severity | FEV₁ Percentage Predicted |
| GOLD 1 | Mild | FEV₁ >80 per cent predicted |
| GOLD 2 | Moderate | 50 per cent < FEV₁ <80 per cent predicted |
| GOLD 3 | Severe | 30 per cent < FEV₁ <50 per cent predicted |
| GOLD 4 | Very severe | FEV₁ <30 per cent predicted |
TABLE 1: GOLD (2022) classification based on FEV1
| SCALE | SEVERITY OF DYSPNOEA |
|---|---|
| 0 | No breathlessness except with strenuous exercise |
| 1 | Shortness of breath when hurrying on the level or walking up a slight hill |
| 2 | Walks slower than people of the same age on the level because of breathlessness or has to stop for breath when walking at own pace on the level |
| 3 | Stops for breath after walking about 100 metres or after a few minutes on the level |
| 4 | Too breathless to leave the house or breathless when dressing or undressing |
TABLE 2: MRC dyspnoea scale
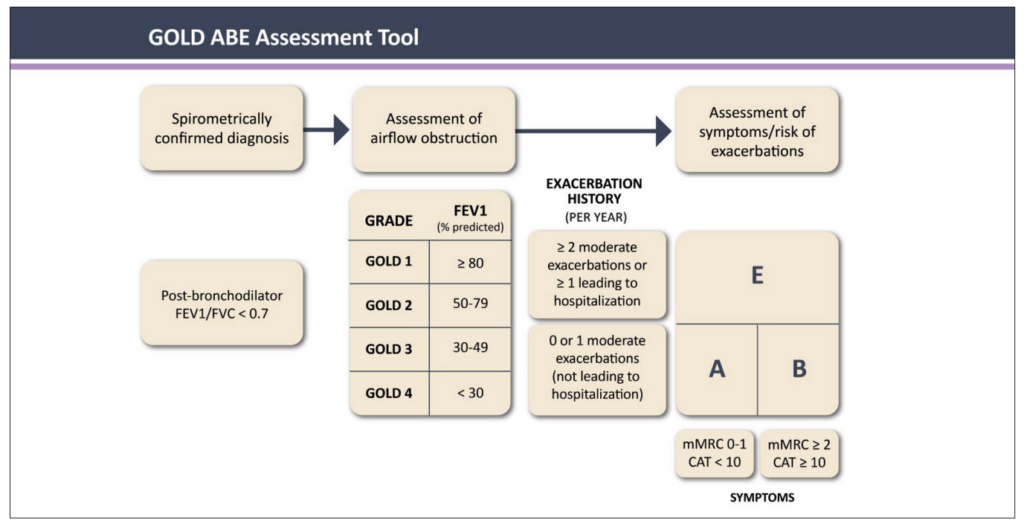
FIGURE 1: The GOLD ABE Assessment Tool¹
Diagnosis
The diagnosis of COPD involves a detailed history, spirometry, investigations, and assessment of symptoms. The history includes:
- Medical and surgical history;
- Smoking history including pack-year history;
- Occupational history.
Spirometry remains the gold standard for an objective assessment of lung function. Post-bronchodilator FEV1/FVC ratio (forced expiratory volume in the first one second to the forced vital capacity ratio) of 70 per cent or 0.7 with bronchodilator reversibility of FEV1 less than 10 per cent is indicative of COPD. Table 1 illustrates the Global Initiative for Chronic Obstructive Lung Disease (GOLD) spirometric classification of severity of obstruction using FEV1.
A full blood screen – including full blood count and thyroid function tests – and electrocardiogram should be performed to rule out comorbidities. A chest x-ray should also be undertaken to rule out conditions such as lung cancer and left-sided heart failure.
The measurement of fractional inhaled nitrous oxide (FENO) is a relatively new concept and is now recommended as one of the tools to assess if inhaled corticosteroid therapy (ICS) is indicated for these patients. Approximately 40 per cent of patients with COPD have raised eosinophils. Patients with high eosinophil levels have an increased risk of exacerbations of COPD if they are not on ICS therapy.
Assessment of symptoms
The characteristic symptoms of COPD are chronic and progressive dyspnoea, cough, and sputum production that can be variable from day to day. Dyspnoea is usually progressive, persistent, and characteristically worse with exercise. Patients may have an intermittent cough which may be unproductive, but many patients will commonly cough up white/clear non-purulent sputum. Symptoms and their impact on quality-of-life can be assessed using the COPD Assessment Tool (CAT test) and the Medical Research Council (MRC) Dyspnoea Scale (Table 2).
The CAT test is a validated eight-item measure of health status impairment in COPD (http://catestonline.org). The maximum score is 40. Scores over 10 are deemed to have a significant impact on the patient’s quality-of-life.
Classification of COPD
Once the objective assessment of COPD is made, COPD can be classified using the GOLD ABE Assessment Tool (Figure 1).¹ This assessment tool includes spirometric assessment and groups patients based on symptoms (primarily breathlessness) and recent history of exacerbations (as an indicator of future exacerbation risk). The classification of COPD at diagnosis is helpful for ensuring the patient is commenced on the correct pharmacological treatment.
Management of COPD
The management of COPD involves both pharmacological and non-pharmacological approaches.
Pharmacological interventions
Bronchodilator therapy remains the mainstay of the management of stable COPD and has been shown to reduce hyperinflation. Pharmacological therapies are used to reduce symptoms, reduce the severity and frequency of exacerbations, and improve exercise tolerance and health status. Combining bronchodilators may increase the degree of bronchodilation whilst lowering the risk of side-effects compared to increasing the dose of a single bronchodilator agent. The main groups of medications include:
- Beta-agonists: These drugs relax smooth muscle by stimulating the beta-2 adrenergic receptors. Beta-agonists can be classified into short-acting (SABA) and long-acting (LABA). Examples include salbutamol (SABA), salmeterol (LABA), indacaterol (LABA), vilanterol (LABA), formoterol (LABA), and olodaterol (LABA).
- Antimuscarinic drugs block the bronchoconstrictor effects of acetylcholine on M3 muscarinic receptors. These can also be classified into short-acting (SAMA) and long-acting (LAMA). Examples include ipratropium (SAMA), tiotropium (LAMA), umeclidinium (LAMA), aclidinium bromide (LAMA), and glycopyrronium (LAMA).
- ICS should not be used as a single agent in the management of COPD. In patients with moderate to severe COPD, the use of ICS combined with a LABA is more effective in improving lung function and health status, and reducing exacerbations, than using either agent alone. Their use in patients with high eosinophil levels have been shown to be beneficial.
Initial pharmacological treatment is based on ABE grouping (Figure 2).¹ The recommendation for patients with a low exacerbation risk and a low symptom burden (CAT <10) (Group A) is a short-acting bronchodilator. For those with a high symptom burden (CAT >10) and a low exacerbation risk (Group B), a LABA or LAMA is recommended. For patients with a high exacerbation risk (Group E), a combination of LABA and LAMA with or without ICS is recommended.
The treatment for patients with a low risk of exacerbation and low symptom burden (Group A) remains the same as previous guidance (Figure 2). The GOLD 2024 report is very clear that there is no longer a role for the LABA + ICS combination for the initial treatment of patients with COPD at low risk for exacerbations.1,6
For patients at high risk for exacerbations, initial treatment is a LABA + LAMA combination (Figure 2). For these patients, a more rational approach to ICS use is recommended, guided by clinical factors and blood eosinophil levels. Patients that are unlikely to benefit from an ICS are those with a blood eosinophil count of 300 cells/µL, a history of hospitalisation for COPD exacerbations with ≥2 moderate exacerbations a year, or with a history of concomitant asthma.
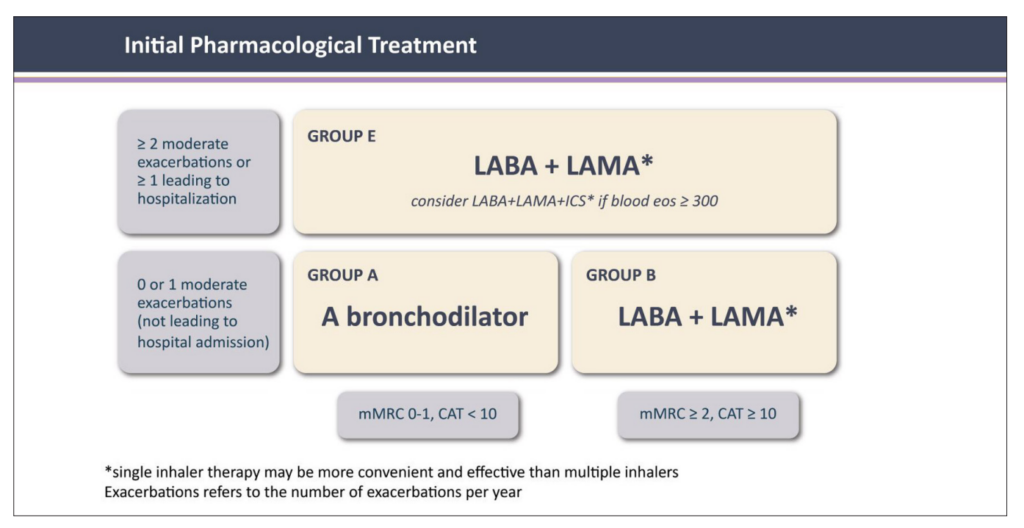
FIGURE 2: Initial pharmacological treatment¹
When considering starting an ICS, blood eosinophils are not the only useful factor. There are known harms of ICS use, including an increased risk of pneumonia and of mycobacterial infection.⁶ Patients with a history of recurrent pneumonia and those with a previous mycobacterial infection should not be routinely started on ICS as the harms may well outweigh the benefits. These fundamental changes to the classification and initial treatment of patients with a high risk of future exacerbations reflect the findings of the ECLIPSE study. This study demonstrated that an eosinophil count, an indicator of underlying inflammation, was a better predictor of response to ICS therapy than a high symptom burden.
Management of patients with ongoing symptoms or exacerbations
The rational approach to the use of ICS therapy based on evidence of an underlying inflammatory process, greatly simplifies both the approach to initial treatment and the follow-up treatment decisions (Figure 3).¹ The first step for any patient with ongoing symptoms or repeated exacerbations is to review and optimise their current treatment regimen – check inhaler technique, check adherence to treatment, and consider whether any comorbid conditions are present or require review.
Subsequent steps depend on whether the patient has ongoing breathlessness or repeated exacerbations (treatable traits), regardless of their initial grouping (Figure 3). Patients with ongoing breathlessness who are receiving bronchodilator monotherapy can be escalated to combination LABA + LAMA therapy.¹ For those already on combination therapy, switching to an alternative device or molecule can be considered alongside a focus on treatment optimisation, ie, adherence and inhaler technique.
Non-pharmacological management such as pulmonary rehabilitation and investigation of alternative causes of breathlessness should also be explored. Patients with ongoing exacerbations can be escalated to triple therapy including an ICS if eosinophils are elevated >300 cells/µL (if not contraindicated).
Triple therapy with the addition of ICS can subsequently be offered for patients who experience a severe exacerbation (requiring hospitalisation) or who experience two moderate exacerbations within a year. If it is not clear which treatable trait (dyspnoea or exacerbations) predominates, it is recommended to follow the pathway for exacerbations.
Inhaler technique
The assessment of inspiratory effort is important to ensure that the patient has sufficient inspiratory flow to optimise deposition of medication to the lungs. Inspiratory effort can be assessed using the in check dial meter. Inhaler devices have different inspiratory flow requirements, with dry powder devices requiring a greater effort than soft mist or meter dose inhalers. To improve technique, it is recommended that patients are educated and trained with the appropriate devices. The choice of device should be tailored to the individual depending on the patient’s ability to use it, their manual dexterity, cognitive function, and preference.
Vaccinations: In addition to influenza, pneumococcal, and Covid-19 vaccines, the Center for Disease Control and Prevention (CDC) recommends the Tdap vaccine (pertussis, tetanus, and diphtheria) for COPD patients who were not vaccinated in adolescence, as well as routine use of shingles vaccine in all COPD patients over 50 years.
Non-pharmacological management
Smoking cessation: Smoking cessation is of paramount importance in the management of COPD, regardless of disease severity. Support given by health professionals significantly increases quit rates over self-initiated strategies. Even a brief (three-minute) period of counselling to urge a smoker to quit results in smoking quit rates of 5-10 per cent. Nicotine replacement therapy (nicotine gum, nasal spray, transdermal patch, sublingual tablet, or lozenge), as well as treatment with varenicline, reliably increases long-term smoking abstinence rates and both are significantly more effective than placebo.¹
Pulmonary rehabilitation: Pulmonary rehabilitation has been proven to show significant benefits in reducing dyspnoea, fatigue, and exacerbations, and improving quality-of-life in people with COPD. Although an effective pulmonary rehabilitation programme is six weeks, the longer the programme continues, the more effective the results. If exercise training is maintained at home, the patient’s health status generally remains above pre-rehabilitation levels.⁸
Exercise: Patients should be encouraged to be as physically active as possible. The aim is to achieve 30 minutes of aerobic activity daily (eg, walking, cycling, swimming). Exercises such as chest-and-shoulder exercises, shoulder raises, step-ups, and sit-to-stand exercises should be encouraged as this will assist in maintaining upper body strength with the ultimate of prevention of muscle wasting.
Breathing exercises such as pursed lip breathing and the active cycle of breathing can help manage dyspnoea and assist in expectorating sputum. These techniques are available on www.copd.ie. COPD
Support Ireland (COPDSI) provides a SingStrong project which delivers interventions to address the biopsychosocial problems through community-based singing and breathing classes, education, and social interaction. The programme is delivered online (www.copd.ie/singstrong/). Energy conservation is based on the 4Ps – prioritise, pacing, planning, and posture – which can be encouraged to assist with daily activities.
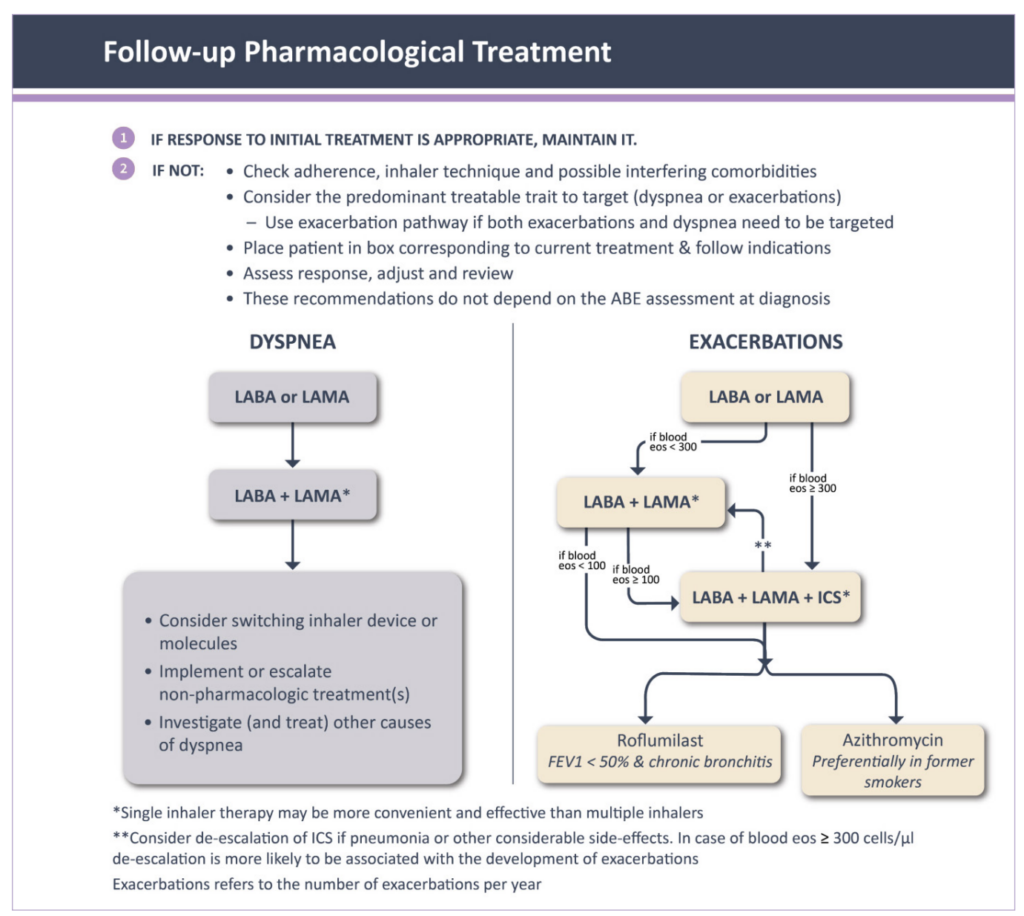
FIGURE 3: Follow-up pharmacological treatment¹
Education: Patients require ongoing education and support to assist them to live and maintain optimal lifestyles. COPDSI provide support groups nationwide where patients can learn about COPD and participate in exercise programmes. Information and contact details for the groups are available on www.copd.ie. Education about the disease process, inhaler technique, adherence to medication, immunisations, pulmonary rehabilitation, smoking cessation, and long-term oxygen therapy are required. All patients should have a self-management plan which outlines their treatment and gives guidance on how to recognise worsening disease.
Oxygen therapy: Long-term oxygen therapy is indicated for stable patients who have:
- PaO2 at or below 7.3kPa (55mmHg) or SaO2 at or below 88 per cent, with or without hypercapnia confirmed twice over a three-week period;
- Or PaO2 between 7.3kPa (55mmHg) and 8.0kPa (60mmHg), or SaO2 of 88 per cent, if there is evidence of pulmonary hypertension, peripheral oedema suggesting congestive cardiac failure, or polycythaemia (haematocrit >55 per cent).
Once placed on long-term oxygen therapy, the patient should be re-evaluated after 60-90 days with
repeat arterial blood gas (ABG) or oxygen saturation while inspiring the same level of oxygen or room air to determine if oxygen is therapeutic and still indicated, respectively.¹
Management of co-morbidities
Many patients with COPD have co-existing illnesses such as diabetes, cardiovascular disease, osteoporosis, and depression to mention but a few. In general, the presence of comorbidities should not affect COPD treatment and comorbidities should be treated according to standards and guidelines. Lung cancer is common in patients with COPD due to smoking history.
Around 50 per cent of patients who experience exacerbations of their COPD and require hospital admission have undiagnosed cardiovascular disease (CVD).¹ Addressing their CVD reduces subsequent admissions to hospital. It is recommended that these patients should have an echocardiograph, B-type natriuretic peptide (BNP), and cardiac MRI.
Gastroesophageal reflux is common and is associated with an increased risk of exacerbations and therefore should be managed optimally. Osteoporosis is also common due to recurrent use of oral steroids, lack of weight-bearing exercise, smoking, and being over or underweight. GOLD 2024 recommend that treatments for comorbidities should kept as simple as possible to avoid polypharmacy.1
COPD exacerbations
The definition of COPD exacerbation has been revised to “an event characterised by dyspnoea and/or cough and sputum that worsen over <14 days. Exacerbations of COPD are often associated with increased local and systemic inflammation caused by airway infection, pollution, or other insults to the lungs.”¹
Currently, exacerbations are classified after the event has occurred as:
- Mild (treated with short-acting bronchodilators only);
- Moderate (treated with short-acting bronchodilators and oral corticosteroids + antibiotics);
- Severe (patient requires hospitalisation or visits the emergency department). Severe exacerbations may also be associated with acute respiratory failure.¹
Exacerbations result in the loss of lung function which may not reverse, as well as deconditioning and loss of mobility. Every effort should be made to avoid and prevent exacerbations.
Conclusion
Steroid stewardship, both oral and inhaled, remains relevant to avoid exposing patients to treatments that they will not benefit from, and which may in fact place them at risk for side-effects. Regular review and managing patients according to their treatable traits is essential to avoid exacerbations. This allows healthcare professionals to provide a more holistic approach to COPD management as it recognises the trait which is most problematic for the patient and allows treatment to be individualised and more targeted.
While a cure for COPD remains elusive and treatment is largely reactive to clinical presentation, there is much we can do to ensure patients receive treatments that relieve their most impactful daily symptoms, optimise their lung function, and reduce their risk for life-threatening exacerbations.
References
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for prevention, diagnosis, and management of COPD: 2024 Report. US: GOLD; 2024. Available at: www.goldcopd.org/2023-gold-report-2/.
- Health Service Executive. COPD. Dublin: HSE; 2024. Available at: www2.hse.ie/conditions/copd/.
- Health Service Executive. National Clinical Programme for Respiratory End to End COPD Model of Care. Dublin: HSE; 2019. Available at: www.hse.ie/eng/about/who/cspd/ncps/ncpr/copd/moc/.
- Adeloye D, Song P, Zhu Y, et al. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: A systematic review and modelling analysis. Lancet Respir Med. 2022;10(5):447-458.
- Çolak Y, Løkke A, Marott JL, et al. Low smoking exposure and development and prognosis of COPD over four decades: A population-based cohort study. Eur Respir J. 2024;64(3):2400314.
- Banerji D, Mahler DA, Hanania NA. Efficacy and safety of LABA/LAMA fixed dose combinations approved in the US for the management of COPD. Expert Rev Respir Med. 2016;10:767-80.
- Faner R, Tal-Singer R, Riley JH, et al. Lessons from ECLIPSE: A review of COPD biomarkers. Thorax. 2014;69:666-72.
- McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;2015(2):CD003793.
- Centres for Disease Control and Prevention. Post-Covid conditions: Information for healthcare providers. USA: CDC; 2024. Available at: www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html.
- Steer J, Kibbler DP, Bourke SC, et al. Undiagnosed and undertreated heart disease in hospitalised patients with exacerbation of COPD. European Respiratory Society oral presentation. Barcelona: ERS; 2022.











Leave a Reply
You must be logged in to post a comment.