The introduction of new molecular technology to the National Newborn Bloodspot Screening Laboratory (NNBSL) is required to implement screening for severe combined immunodeficiency (SCID) and spinal muscular atrophy (SMA), according to the HSE. The Executive indicated this was an additional requirement to the “substantial” body of work required to introduce any new condition to the programme.
In 2023, the then Minister for Health accepted the recommendations of the national screening advisory committee to add SCID and SMA to the National Newborn Bloodspot Screening Programme (NNBSP). In 2024, €1.4 million in new development funding was allocated to support the expansion of the programme.
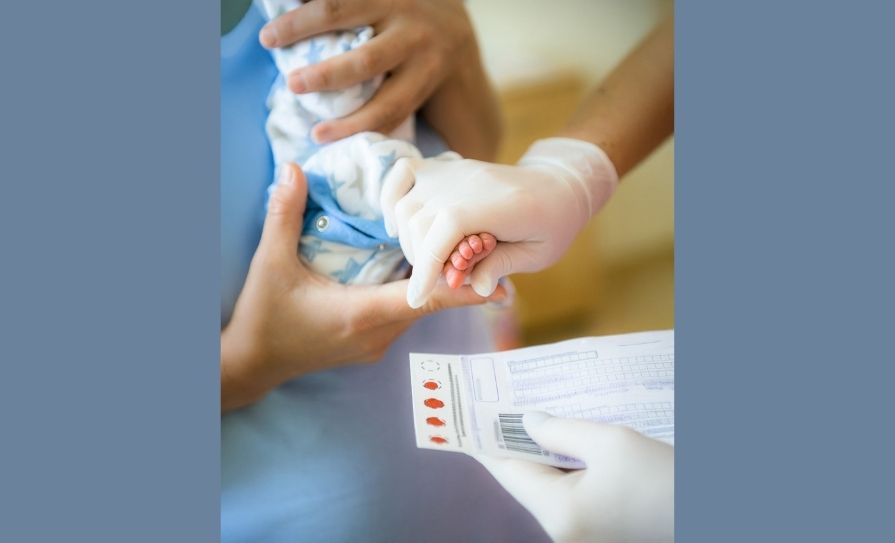
Reviews conducted by HIQA illustrated the importance of early identification of these rare, but serious genetic disorders to allow for early diagnosis and treatment and improved outcomes.
A HSE spokesperson told the Medical Independent the molecular technology needed for SMA and SCID screening requires procurement of specialised equipment and recruitment and training of additional staff.
“The HSE and the NNBSP needs to ensure that the introduction of screening for any new condition is conducted with sufficient rigour to ensure a safe, quality assured service that meets all relevant laboratory and clinical accreditation standards.”
The spokesperson noted that health technology assessments undertaken by HIQA “highlighted the fact that screening may not be feasible in advance of the move of the NNBSL to the new National Children’s Hospital due to infrastructural constraints”. However, the NNBSP was “committed to supporting implementation of screening in a timely manner, pending confirmation of the final completion date for the new National Children’s Hospital”.
The NNBSP and NNBSL will commence preparatory work at the Temple Street site, including the validation and verification of the methodology. This preparatory work requires structural reconfiguration of the laboratory space to accommodate the new equipment and additional staff.
“The required laboratory staff have been recruited and are in post. Staff have also been recruited at programme level/within the National Healthy Childhood Programme to lead and project manage the implementation of screening for SCID/SMA and to continue to keep delivering the quality assurance of the current NNBSP.”
The tender to procure the required laboratory equipment has been completed and the equipment will be ordered for delivery in the coming weeks. The structural building reconfiguration works are ongoing. It is hoped that they will be also completed “in the coming weeks and the verification of the screening methodology will then commence”.
“As regards timelines, the work is ongoing to estimate how long the laboratory verification will take, the relevant ICT systems to be reconfigured and all the relevant clinical referral pathways agreed and in place…. The HSE is committed to progressing the implementation of these additional conditions as soon as possible while also ensuring that the expanded programme will be robust, verified, safe, effective and meets the standards expected by parents.”












Leave a Reply
You must be logged in to post a comment.