Award-winning Irish research has identified new therapeutic targets for the treatment of high-grade serous ovarian cancer
Ovarian cancer is one of the most common types of cancer in women, with approximately 400 women diagnosed in Ireland each year (www.ncri.ie). While there has been significant progress in the treatment of some cancers in the past decade, this has not been the case for ovarian cancer, which continues to have a low five-year survival rate of 30 per cent and just 17 per cent for stage 4 patients.
A key reason for these dismal survival rates is the late stage at which most patients are diagnosed, with over half of patients being diagnosed at stage 3 or stage 4, after the cancer has metastasised beyond the site of the primary tumour. It is estimated that cancer metastasis accounts for 90 per cent of all cancer-related deaths,1 and so the high incidence of metastasis in ovarian cancer is a major clinical challenge.
Cytoreductive surgery with HIPEC (hyperthermic intraperitoneal chemotherapy) when indicated and platinum-based systemic chemotherapy, followed by maintenance bevacizumab (anti-VEGF) and/or PARP inhibitors are the main treatment strategies for advanced ovarian cancer. However, recurrences are very common, and so there is urgent need for new therapies.2 Several phase 3 clinical trials testing the efficacy of checkpoint inhibitors yielded poor results, with very low response rates reported.3,4 Moreover, after the disappointing results of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) trial in 2021, where annual serum CA125 measurements plus ultrasound screenings had no impact on ovarian cancer deaths,5 it is crucial that we keep exploring new, innovative ways to diagnose and treat this disease.
The next frontier
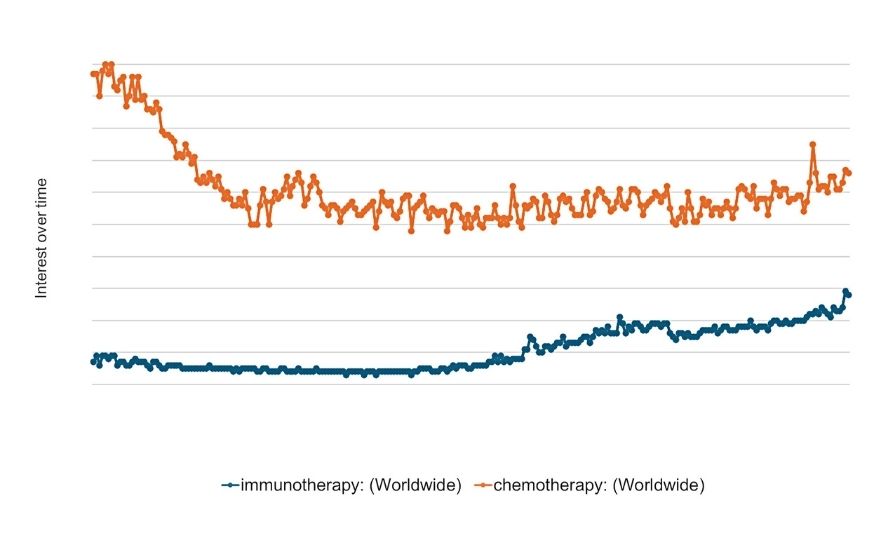
Figure 1: The worldwide growing interest in immunotherapy. Numbers represent Google search interest relative to the highest point on the chart for the given region and time. A value of 100 is the peak popularity for the term, while a value of 50 means that the term is half as popular.
Data source: Google Trends (www.google.com/trends)
Immunotherapy represents the next frontier in cancer therapies and has revolutionised the way certain cancers are treated. Indeed, the approval of the first checkpoint inhibitor ipilimumab (anti-CTLA4) for the treatment of metastatic melanoma in 2011 ushered in this new era in cancer treatment,6 and even garnered the pioneering research scientists, James P Allison and Tasuku Honjo, the Nobel Prize in Physiology or Medicine in 2018. Today, immunotherapy is widely acknowledged as a core pillar of cancer treatment, and advancements and interest in this field are growing (Figure 1). Aside from checkpoint inhibitors and monoclonal antibodies, we also now have US FDA-approved adoptive cell therapies such as chimeric antigen receptor (CAR) T-cells and tumour-infiltrating lymphocyte (TIL) therapy for treating blood cancers,7 some of which have been available to eligible patients at St James’s Hospital, Dublin since 2021.
While great strides have been made in getting these immunotherapies into the clinic, we are only at the early stages of harnessing the full potential of the immune system to treat cancer, and a better understanding of the immunology of human cancer will undoubtedly drive the development of next-generation immunotherapies.
Challenges
A crucial factor that limits the efficacy of immunotherapies is the hostile tumour microenvironment (TME).8 Immunosuppressive features of the TME include high levels of immunomodulatory cytokines such as TGF-β, hypoxia, altered nutrient availability, and the presence of pro-tumourigenic immune subsets such as myeloid-derived suppressor cells. Ovarian cancer often has the added complication whereby patients develop large volumes of pathogenic fluid in the abdomen, known as ascites. This ascites milieu is analogous to a liquid TME, containing altered levels of cytokines and chemokines and has a strong suppressive impact on anti-tumour immune responses.9-12 Moreover, this pro-tumourigenic ascites fluid surrounds the organs within the abdomen and is circulated around the cavity by movements of the diaphragm. It is not surprising therefore that development of ascites is associated with increased disease burden, treatment resistance, and worse prognosis in ovarian cancer.13-15 Indeed, it is possible that the strong immunosuppressive impact of ascites contributes to the low response rates observed with checkpoint inhibitors in ovarian cancer patients.
Study details
While the impact of ascites on anti-tumour immune cells is well described,12,16-19 the mechanisms underlying this are poorly understood. In this study, we endeavoured to explore the molecular mechanisms underlying immune dysfunction in patients with ovarian cancer, with the goal of identifying new therapeutic targets that could be used to unleash the anti-cancer immune response in these patients, and in doing so eradicate the vast metastatic disease that they experience. We first analysed the function and metabolism of natural killer (NK) cells and T-cells isolated from tumours and ascites of treatment-naive high-grade serous ovarian cancer (HGSOC) patients and observed profound immune dysfunction at all sites of disease, including in tumours of the ovary, omentum, peritoneum and lymph node, and in the ascites fluid. To study this dysfunction in more depth, we developed an in vitro model, wherein immune cells from the blood of healthy donors were cultured in increasing concentrations of patient-derived ascites, and this recapitulated the dysfunction observed in tumour- and ascites-infiltrating NK and T-cells.
We hypothesised that certain metabolites in the ascites might be causing the dysfunction in the immune cells. We carried out metabolomic analysis on matched plasma and ascites fluid from HGSOC patients and observed a striking enrichment in many types of lipids in ascites, while the levels of glucose and glutamine, important nutrients for optimal immune responses,20-22 were normal. We next examined the intracellular metabolites in NK cells that had been cultured in ascites and observed a profound enrichment in lipid species. Analysis of cell culture supernatants revealed that the NK cells were taking up lipids from the ascites fluid (Figure 2) – this was an unexpected finding that has not been described before. Depleting the ascites fluid of lipids abrogated its immunosuppressive impact on NK and T-cells, while treating control NK cells with certain ascites-associated lipids, in particular phosphatidylcholine 36:1, recapitulated the dysfunctional phenotype. These experiments showed that lipids in ascites are a key driving factor of NK and T-cell dysfunction in malignant ascites.
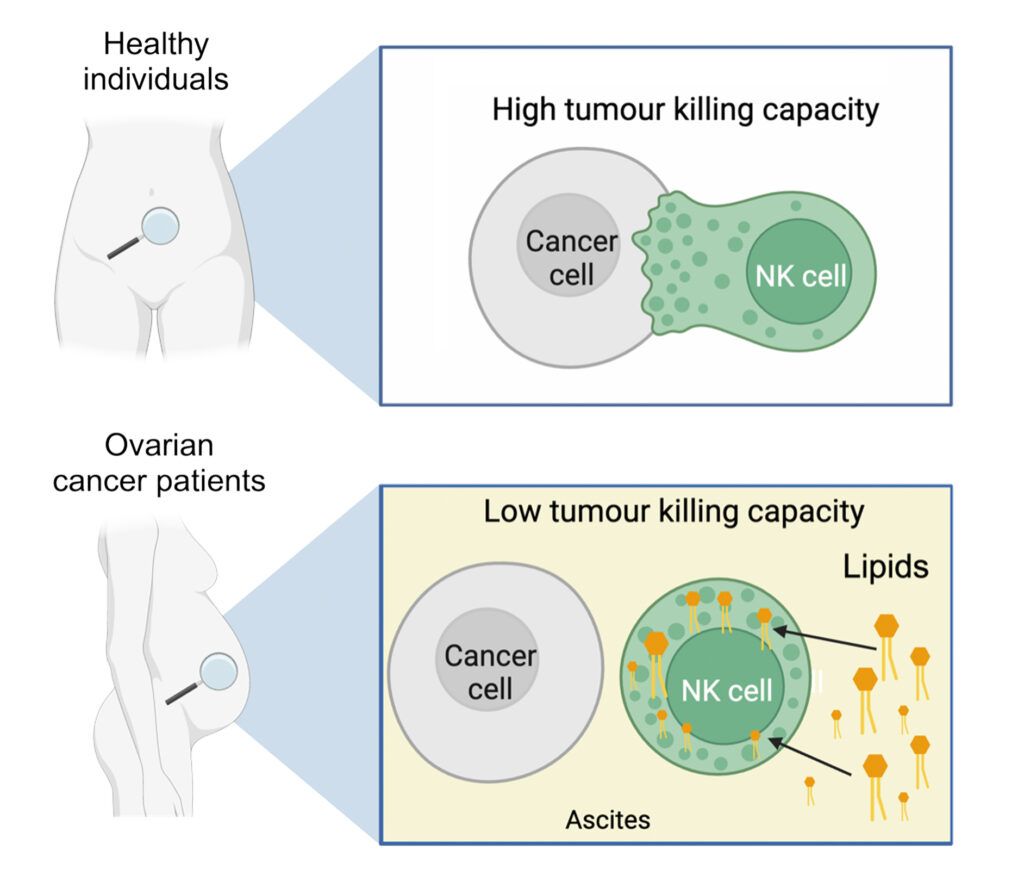
Figure 2: Uptake of lipids from malignant ascites restricts NK cell cytotoxicity
We next aimed to figure out how the lipids were getting into the immune cells. We compared the gene expression levels of many lipid transporters in NK cells isolated from patient ascites or from blood. While many transporters were increased in NK cells from ascites, SCARB1 (scavenger receptor class B type 1) had the greatest fold increase, showing that NK cells turn on SCARB1 transcription in ascites. We hypothesised that blocking SR-B1 (protein encoded by SCARB1) might protect the NK cell functions in ascites. Remarkably, culturing NK cells in ascites in the presence of an antibody blocking SR-B1 protected their capacity to kill tumour cells in vitro. Together, the data show that uptake of lipids, including phosphatidylcholine 36:1, from ascites restricts the cytotoxicity of NK cells against tumour cells, and identifies the lipid transporter SR-B1 as an exciting therapeutic target for advanced HGSOC.
It will be important in future work to test these findings in an in vivo model of ovarian cancer and, if data are promising, in human clinical trials. Targeting SR-B1 using monoclonal antibody and small molecular inhibitors has been effective in murine models of hepatitis C virus infection,23,24 however, its in vivo efficacy against cancer remains unexplored. While our findings suggest that blocking SR-B1 may be an effective therapeutic strategy for boosting the immune system in advanced HGSOC patients, what is more exciting is that we anticipate that this approach may also have the added benefit of starving the tumour cells of lipids, which they use as a critical fuel source.25,26 Indeed, it is the lipid rich environment of the omentum where the primary tumour initially metastasises to in most cases, and we speculate that the omentum may be the source of the pathogenic lipids we identified in patient-derived ascites. If true, identifying the mechanisms that trigger the omentum to start shedding these immunosuppressive lipids into the abdominal cavity will be of high interest and may reveal novel therapeutic targets.
From bench to bedside
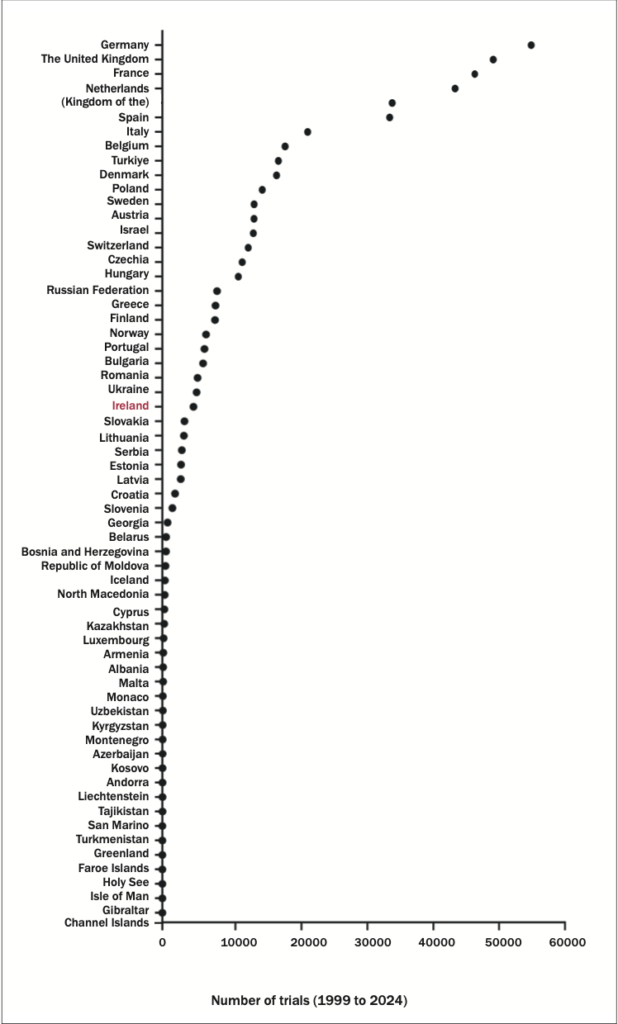
Figure 3: The number of clinical trials in European countries between 1999 and 2024. The number of trials listed in the World Health Organisation (WHO) International Clinical Trials Registry Platform (ICTRP) Note that the ICTRP comprises both interventional and observational trials. Data source: WHO (www.who.int/observatories/global-observatory-on-health-research- and-development/monitoring/number-of-clinical-trials-by-year-country-who-region-and- income-group)
In this ever-evolving era of immunotherapy, it is more important than ever for Ireland to increase its clinical trial activities so that basic research like ours can be translated from bench to bedside. Work from the Cancer Groundshot initiative provided unequivocal evidence that clinical outcomes are superior if patients are treated in research active institutions.27
However, we have a poor history of conducting clinical trials in Ireland. Between the years of 1999 and 2024, we conducted just 4637 – compared to 186,497 and 49,145 in US and UK, respectively (Figure 3). We also conduct far fewer clinical trials than Denmark or Finland, both of which have similar populations and economic performances to Ireland (www.ipha.ie). With new treatments emerging more regularly, increasing equitable access to clinical trials will give Irish patients access to the latest therapies and decrease costs for the exchequer. The National Clinical Trials Oversight Group (NCTOG), responsible for developing a framework to increase the number of interventional clinical trials in Ireland, was established in July 2024, and it is hoped that recommendations from the NCTOG will allow the Government to increase funding, resources and supports available to industry and clinical sites, and promote increased clinical trial activity across the Irish healthcare system.
A more hopeful future
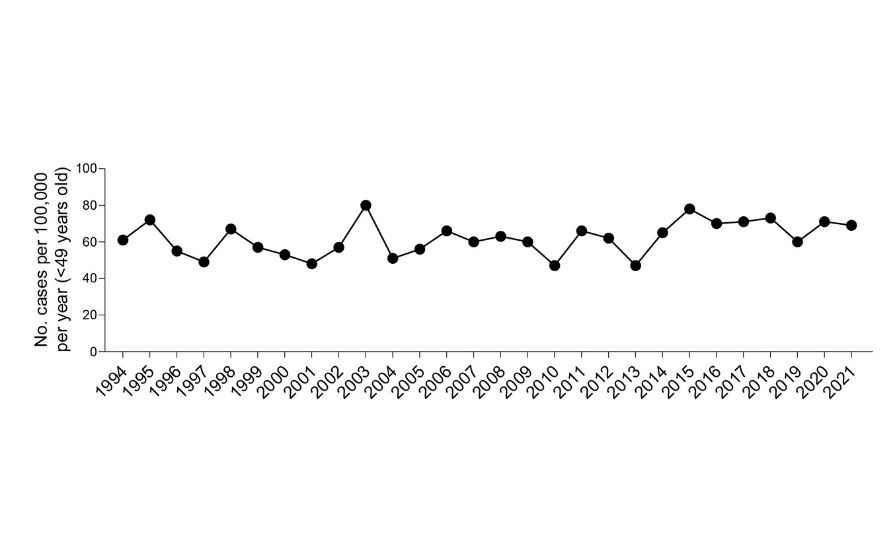
While the prognoses of ovarian cancer patients remain poor today, there are many reasons to be hopeful that this will change in the future. A recent report by clinicians at the Mater Misericordiae University Hospital and St Vincent’s University Hospital in Dublin, showed that improved surgical approaches increased the three-year survival rate of stage 3 and 4 ovarian cancer patients from 35.5 to 76 per cent.28 Moreover, the explosion in immunotherapy research, such as ours, will likely remodel the way we treat cancer in the future and result in creative solutions for targeting cancer using the patient’s immune system, or possibly even the immune system of someone else. Despite the alarming recent study showing that cancer is increasing in young people,29 particularly in women, this does not appear to be the case for ovarian cancer in Ireland (Figure 4), and case numbers appear steady. As Ireland currently spends just ~1 per cent of our gross domestic product on research (less than half of the European average of ~2.3 per cent and much lower than the EU target of 3 per cent, ec.europa.eu), increasing funding and resources for basic scientific research and clinical trials is paramount and will undoubtedly turn the tide for ovarian cancer and return innovative treatments for these patients.
Acknowledgements
The work described here was funded by the Irish Cancer Society immuno-oncology grant, without which the research would not have been possible. The project was supervised by Prof Lydia Lynch, Professor of Molecular Biology, Princeton University, US, and Prof Donal Brennan, Consultant Gynaecological Oncologist, Mater Misericordiae Hospital, Dublin, Professor of Gynae-Oncology, University College Dublin, and National Lead for Cancer Research at the NCCP and Chairperson of the NCTOG.
References
1. Guan X. Cancer metastases: Challenges and opportunities. Acta Pharm Sin B. 2015;5(5):402-18
2. Kurnit KC, Fleming GF, Lengyel E. Updates and new options in advanced epithelial ovarian cancer treatment. Obstet Gynecol. 2021;137(1):108-21
3. Matulonis UA, Shapira-Frommer R, Santin AD, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: Results from the phase II KEYNOTE-100 study. Ann Oncol. 2019;30(7):1080-7
4. Randall LM, O’Malley DM, Monk BJ, et al. Niraparib and dostarlimab for the treatment of recurrent platinum-resistant ovarian cancer: Results of a phase II study (MOONSTONE/GOG-3032). Gynecol Oncol. 2023;178:161-9
5. Menon U, Gentry-Maharaj A, Burnell M, et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet. 2021;397(10290):2182-93
6. Alexander W. The checkpoint immunotherapy revolution: What started as a trickle has become a flood, despite some daunting adverse effects; new drugs, indications, and combinations continue to emerge. P T. 2016;41(3):185-91
7. World’s first TIL therapy approved. Nat Biotechnol. 2024;42:349
8. Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12(1):31-46
9. Zheng X, Wang X, Cheng X, et al. Single-cell analyses implicate ascites in remodeling the ecosystems of primary and metastatic tumors in ovarian cancer. Nat Cancer. 2023 Aug;4(8):1138-1156
10. Matte I, Lane D, Laplante C, et al. Profiling of cytokines in human epithelial ovarian cancer ascites. Am J Cancer Res. 2012;2(5):566-80
11. Shender VO, Pavlyukov MS, Ziganshin RH, et al. Proteome-metabolome profiling of ovarian cancer ascites reveals novel components involved in intercellular communication. Mol Cell Proteomics. 2014;13(12):3558-71
12. Kilgour MK, MacPherson S, Zacharias LG, et al. 1-Methylnicotinamide is an immune regulatory metabolite in human ovarian cancer. Sci Adv. 2021;7(4)
13. Nagy JA, Herzberg KT, Dvorak JM, et al. Pathogenesis of malignant ascites formation: Initiating events that lead to fluid accumulation. Cancer Res. 1993;53(11):2631-43
14. Asem M, Young A, Oyama C, et al. Ascites-induced compression alters the peritoneal microenvironment and promotes metastatic success in ovarian cancer. Sci Rep. 2020;10(1):11913
15. Kipps E, Tan DS, Kaye SB. Meeting the challenge of ascites in ovarian cancer: New avenues for therapy and research. Nat Rev Cancer. 2013;13(4):273-82
16. Fraser CC, Jia B, Hu G, et al. Ovarian cancer ascites inhibits transcriptional activation of NK cells partly through CA125. J Immunol. 2022;208(9):2227-38
17. Foord E, Arruda LCM, Gaballa A, et al. Characterisation of ascites- and tumor-infiltrating T-cells reveals distinct repertoires and a beneficial role in ovarian cancer. Sci Transl Med. 2021;13(577)
18. Song M, Sandoval TA, Chae CS, et al. IRE1α-XBP1 controls T-cell function in ovarian cancer by regulating mitochondrial activity. Nature. 2018;562(7727):423-8
19. Poznanski SM, Singh K, Ritchie TM, et al. Metabolic flexibility determines human NK cell functional fate in the tumour microenvironment. Cell Metab. 2021;33(6):1205-22.e5
20. Assmann N, O’Brien KL, Donnelly RP, et al. Srebp-controlled glucose metabolism is essential for NK cell functional responses. Nat Immunol. 2017;18(11):1197-206
21. Ma EH, Verway MJ, Johnson RM, et al. Metabolic profiling using stable isotope tracing reveals distinct patterns of glucose utilisation by physiologically activated CD8(+) T-cells. Immunity. 2019;51(5):856-70.e5
22. Ma EH, Dahabieh MS, DeCamp LM, et al. (13)C metabolite tracing reveals glutamine and acetate as critical in vivo fuels for CD8 T-cells. Sci Adv. 2024;10(22):eadj1431
23. Vercauteren K, Van Den Eede N, Mesalam AA, et al. Successful anti-scavenger receptor class B type I (SR-BI) monoclonal antibody therapy in humanised mice after challenge with HCV variants with in vitro resistance to SR-BI-targeting agents. Hepatology. 2014;60(5):1508-18
24. Syder AJ, Lee H, Zeisel MB, et al. Small molecule scavenger receptor BI antagonists are potent HCV entry inhibitors. J Hepatol. 2011;54(1):48-55
25. Nieman KM, Kenny HA, Penicka CV, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumour growth. Nat Med. 2011;17(11):1498-503
26. Zhao G, Cardenas H, Matei D. Ovarian cancer – why lipids matter. Cancers (Basel). 2019;11(12)
27. Lawler M, Davies L, Oberst S, et al. European Groundshot – addressing Europe’s cancer research challenges: A Lancet Oncology Commission. Lancet Oncol. 2023;24(1):e11-e56
28. Mulligan K, Corry E, Donohoe F, et al. Multidisciplinary surgical approach to increase survival for advanced ovarian cancer in a tertiary gynaecological oncology centre. Ann Surg Oncol. 2024;31(1):460-72
29. Siegel RL, Kratzer TB, Giaquinto AN, et al. Cancer statistics, 2025. CA Cancer J Clin. 2025;75(1):10-45










Leave a Reply
You must be logged in to post a comment.