PROF DOMINIC A HEGARTY, Consultant in Pain Management and Neuromodulation, Mater Private Hospital, Cork; Associate Professor in Pain Medicine, University College Cork; and Clinical Director, Pain Relief Ireland, www.painreliefireland.ie
Diabetic neuropathy (DN) represents a common, disabling and, until recently, largely neglected problem affecting approximately 50 per cent of patients with diabetes at some point. According to the International Diabetes Federation, 425 million people world-wide aged 20 years had diabetes in 2017, and this number is expected to increase to 629 million by 2045. Approximately one-in-three people with diabetes experience painful diabetic neuropathy (PDN).
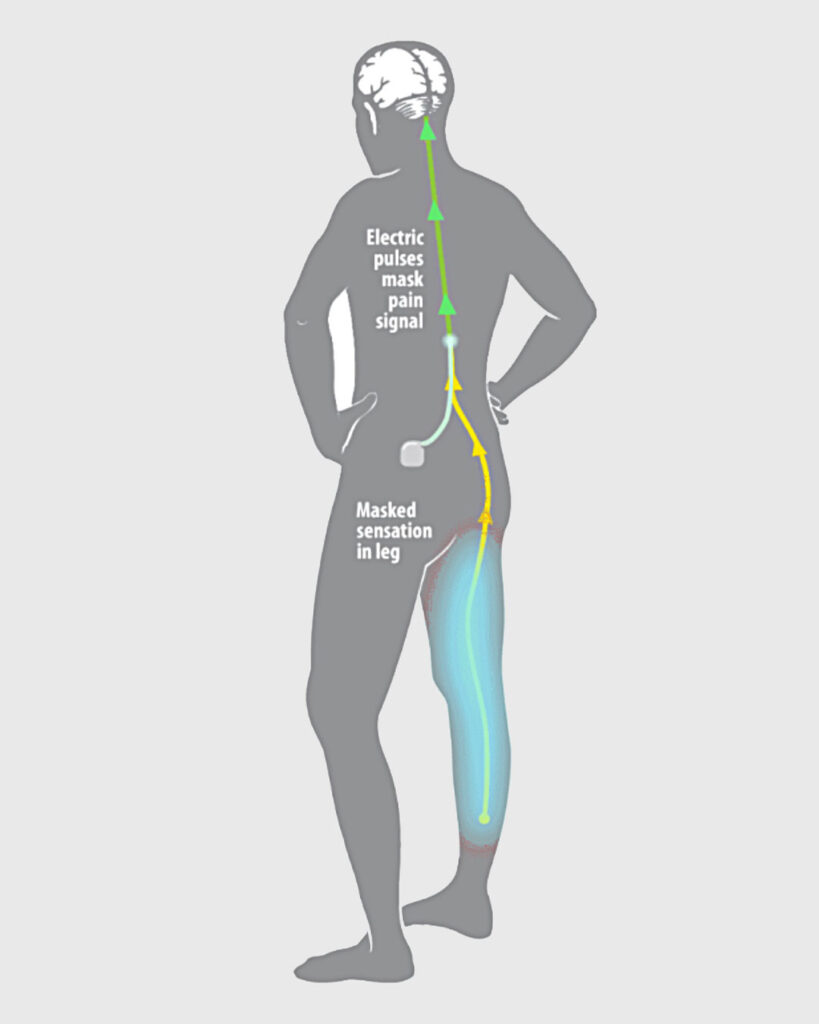
FIGURE 1: SCS uses either (a) low-frequency current to replace the pain sensation with a mild tingling feeling called paraesthesia or (b) high-frequency or burst pulses to mask the pain with no tingling feeling. This inhibits the transmission of painful sensations to the brain based on the ‘Gate Control Theory’ Source: https://mayfieldclinic.com/pe-stim.htm
Ignoring PDN is no longer acceptable because the impact it has on an individual’s quality-of-life once established can lead to several costly complications, including persistent neuropathic pain, ulcers, Charcot foot, and an increased risk of amputations associated with increased mortality. A recent cohort study reported that patients with PDN were twice as likely to use opioids and over 16 times more likely to have an amputation than patients with diabetes mellitus without neuropathy.
Until recently, medical management was the only option available and it would often fail to control the situation. That is likely to change with the recent research and subsequent approval by the US FDA of the use of spinal cord stimulation (SCS) to treat painful diabetic peripheral neuropathy.
Defining diabetic neuropathy
A broad and simple definition of DN is “the presence of symptoms and/or signs of peripheral nerve dysfunction in people with diabetes after the exclusion of other causes”. DN is among other factors attributable to hyperglycaemia, hyperglycaemia-associated metabolic derangement, dyslipidaemia, and micro vessel alterations.
The most common form of DN is a chronic symmetrical length-dependent sensorimotor polyneuropathy, termed diabetic polyneuropathy, which accounts for 75- to-90 per cent of all DN cases. Other types of DN include autonomic neuropathy, diabetic radiculoplexopathy (formerly called diabetic amyotrophy), mononeuropathies, and treatment-induced neuropathies.
The remainder of this review will focus on PDN.
Aetiology of PDN
Painful neuropathy is a common but inconsistent feature of chronic diabetes. Clinical studies have yet to offer robust clues to the pathogenesis of pain in diabetic patients, so there are no targeted prophylactic therapies.
Animal studies in diabetic rodents have suggested that over-expression of sensory receptors and ion channels in primary afferents can exaggerate or modify sensory input from the periphery. This is followed by amplification of sensory processing at the spinal cord level via sensitisation and disinhibition mechanisms.
| Agent | RR | 95% CI | P | 12 |
| Pregabalin | 1.32 | 1.10-1.58 | < 0.003 | 44% |
| Duloxetine | 1.34 | 1.01-2.02 | < 0.004 | 76% |
| Tapentadol | 1.38 | 1.12-1.71 | < 0.003 | 0% |
| Capsaicin | 0.99 | 0.73-1.36 | 0.97 | 0% |
| Gabapentin | 2.39 | 0.57-10.00 | 0.23 | 87% |
TABLE 1: Summary of key medications for treatment of PDN
Source: Jingxuan L, et al (2021). Different Drugs for the Treatment of Painful Diabetic Peripheral Neuropathy: A Meta-Analysis. Front Neurol. 12:682244
There is also an emerging appreciation that the higher nervous system is not spared in diabetes, so that amplifier or generator sites for pain may be located within the brain and project pain sensations to the periphery. The developing appreciation of the pathogenesis of pain in animal models of diabetes has offered mechanistic validations for some therapies in current clinical use, such as gabapentanoids, low-dose lidocaine, and alpha-lipoic acid while also highlighting new sites for intervention that may offer greater specificity and a reduced side-effect profile. It also raises questions regarding the use of bioelectric management to modify the transmission of pain in order to provide early symptom relief.
Diagnosis and management of PDN
Diabetic neuropathies are complex diseases, and no robust definition or gold standard exist that fully encompass the complexity and changing course of nerve fibre damage in PDN. The hierarchical classification of PDN by the Toronto criteria into ‘possible’, ‘probable’ or ‘definite’ addresses this issue by these criteria:
a) Possible PDN requires symptoms of decreased sensation (eg, numbness or pricking feeling in the toes, feet or legs) or signs (ie, symmetric decreased sensation or decreased or absent ankle reflexes).
b) Probable PDN requires symptoms and signs, including two or more of the following: neuropathic symptoms, decreased distal sensation, or decreased or absent ankle reflexes.
c) Definite PDN requires abnormal nerve conduction studies or an abnormal validated measure of small fibre damage in combination with a symptom or a sign. QST added substantial information in this area.
Importantly, these criteria permit for the changing course of nerve fibre damage in PDN, but at the same time they also illustrate the clinical challenge that there is no specific measure to diagnose PDN in any individual at all times throughout the course of PDN.
The examination for PDN starts at the ‘bedside’ with simple assessments of signs of neuropathy (Figure 2). In addition, the examination should include foot inspection, joint mobility testing and evaluation of motor function. A number of scoring systems have been developed for the screening of PDN. Two examples include the Toronto Clinical Neuropathy Scoring System, and the Diabetic Neuropathy Symptom Score.
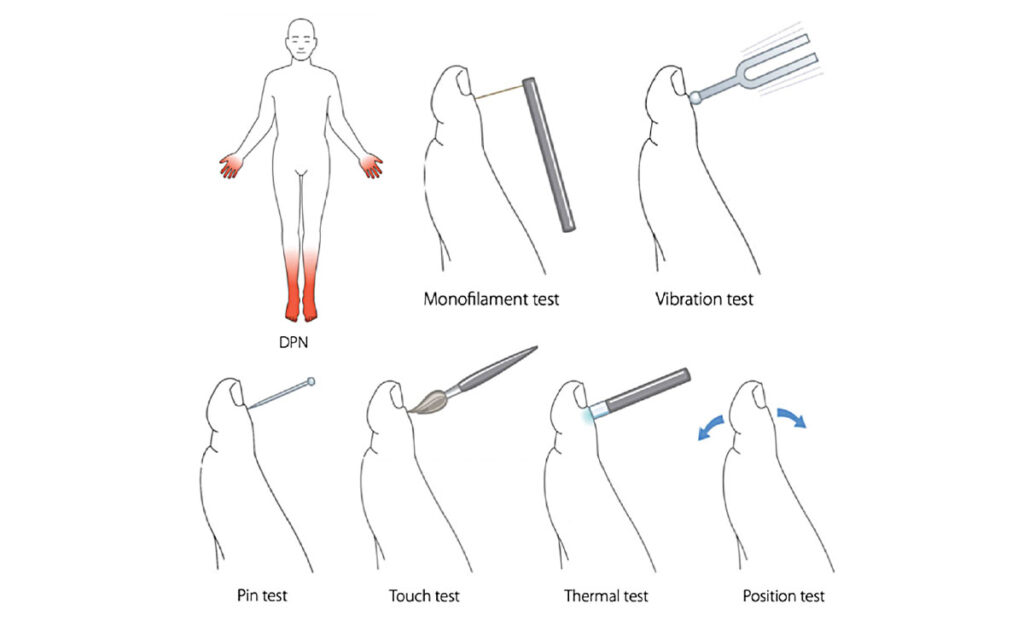
FIGURE 2: Diabetic polyneuropathy (DPN). Beside tools for testing cutaneous sensation, both large fibre function: 10-g monofilaments, vibration with 128-Hz turning fork, touch and joint position, and small fibre function: Cols and warm sensation, and pinprick
Source: Gylfadottir et al. J Diabetes Investig 2019; 10: 1,148-1,157
Present medical management options
Over the past 12 years there have been many reports on the effects of different drugs for the treatment of PDN. A meta-analysis in 2021 where the pain score was used as the main result and 30 per cent and 50 per cent pain reduction and adverse events were used as secondary results, identified 37 relevant studies. The key agents are listed in Table 1. In summary, pregabalin and duloxetine showed good therapeutic effects on painful PDN, but adverse events, which were not unexpected, were also significant. Tapentadol was found to have a good analgesic effect, but due to possible risk of opioid addiction, it needs to be very cautiously approached in clinical use.
The analgesic effects of ABT-894 and gabapentin need to be further studied with longer and larger randomised controlled trials (RCTs). Although lacosamide, mirogabalin, and capsaicin are more effective than placebo, the therapeutic effect is weaker than pregabalin.
Spinal cord stimulation as a treatment option
Neuromodulation or modulation of nerve fibre activity at the spinal cord (SCS) is an established treatment option for neuro pathic pain, such as failed back surgery syndrome and complex regional pain syndrome. In July 2021 the US FDA approved the use of SCS to treat PDN especially for those who have not responded to traditional medical management and in whom their diabetes is under reasonable control. For the first time, individuals with PDN now have a treatment that can have a meaningful improvement on activities of daily living (ADL).
The concept of SCS is that it provides focused electrical current on the dorsal column to counter balance the painful signals thereby providing symptom relief. It does not eliminate the pain source, but it changes the way the brains perceives the pain. The devices themselves can be programmed to influence the pain pathway personalising the therapy (Figure 1).
Evidence of clinical improvement
Overall SCS has shown a mean reduction in pain intensity of 23.13 (95% CI 24.19 to 22.08) for the management of PDN with SCS when compared with basic medical therapy (BMT). Statistically-significant differences were also observed for the proportion of patients achieving at least a 50 per cent pain reduction, EQ-5D utility index, and EQ-5D VAS; all showing beneficial effects of SCS compared with BMT for patients with PDN.
Ability to personalise treatment
The ability of SCS devices to deliver differ types of stimulation patterns is an important advancement. This ability greatly increases the chance of improving outcomes for those with PDN. For example, RCTs evaluating high-frequency SCS at 10kHz for PDN have shown a greater improvement in the proportion of patients with at least a 50 per cent reduction in lower-limb pain without a clinically meaningful neurological deficit compared with baseline at three months (SCS (86 per cent) when compared with patients receiving conventional medical management alone (5 per cent). Encouraging results have also been reported in a case series and a small cross-over RCT evaluating burst SCS for PDN. RCT evidence is required to ascertain the effectiveness of burst SCS for PDN.
The use of conventional SCS parameters should also provide pain control, and the introduction of Differential Targeted Multiplexed (DTM) SCS programming that involves stimulation for the glial cells in the dorsal column could also provide another useful control point in the pain pathway.
Although the effectiveness of conventional paraesthesia inducing SCS for PDN has been demonstrated, its cost-effectiveness has not yet been established. A cost-utility analysis from a societal perspective should be conducted based on the outcomes of SCS.
Planning for the future: 2022 and beyond
Diabetes is expected to increase in the population and it will bring with it an expected increase in diabetic-related complications. PDN is one such predictable complication that needs to be considered. Pro-active management of diabetes has been shown to help reduce long-term complications. These step could include:
a) Screening for early features of PDN should be included in the bi-annual screening programmes presently available. The prevalence of DN often proceeds other complications so regular clinical screening for PDN could be crucial. This would permit the inclusion of suitable analgesics early and before peripheral sensitisation becomes irreversible.
b) An awareness campaign should be also be provided to all primary care centres and to all healthcare providers who manage individuals with diabetes. Actively engaging with patient-focused diabetes groups would be a practical way to highlight the issues and the solutions. Supporting podiatry services with equipment to undertake simple non-invasive sensory assessments (QST) would allow for early and effective identification of those who could benefit from advanced treatments and prevent complications.
c) Commencement of suitable analgesics should be considered sooner. The evidence suggests that refractory cases (ie, those cases who failure to respond to basic medical management) should be considered for SCS as soon as possible. Personalised drug-free technology centred treatment could improve an individual’s quality-of-life by 50 per cent in a matter of weeks.
d) The National Clinical Programme for Diabetes (NCPD) published A Practical Guide to Integrated Type 2 Diabetes Care (2016) outlining the proposed pathway to “integrated” healthcare. While this publication dealt with the management of the diabetic foot and outlined an algorithm of treatment options, unfortunately, management of PDN was not mentioned in the document nor was there any mention of the possibility of a pain management referral as part of the “integrated” approach. Hopefully this can be rectified when the next document is prepared so that the pain management can be included and the new, proven technology-centred therapy options can be recommended and provided in a timely fashion.
Acknowledgement
If you feel you have individuals who could benefit from QST assessment, SCS or other possible treatment options please contact info@painreliefireland.ie for an appointment.









Leave a Reply
You must be logged in to post a comment.