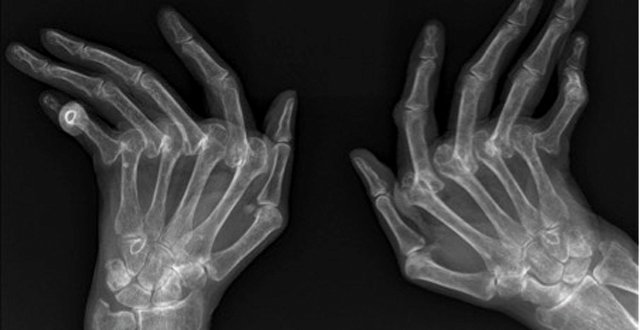
Psoriatic arthritis (PsA) is a chronic inflammatory disease of the musculoskeletal system that, like rheumatoid arthritis (RA), can lead to joint damage, deformity, functional impairment and disability.
However, PsA is a distinct entity from RA, with a differing pattern and distribution of peripheral and axial joint involvement in the majority of cases. Unlike RA, PsA is associated with psoriasis, dactylitis and enthesitis, features of spondyloarthropathy (SpA); up to 30 per cent of patients with psoriasis have evidence of PsA.
SpA has a strong association with the HLA-B27 genotype, and aside from PsA, also includes ankylosing spondylitis, reactive arthritis (formerly known as Reiter’s syndrome) and enteropathic arthritis seen in inflammatory bowel disease.
The aim of modern treatment of PsA is to prevent joint damage, thereby preserving joint function and patient independence. Joint damage in PsA manifests radiographically as articular erosion and/or ankylosis of affected joints, which will be seen clinically as reduction in the normal range of movement of the joint and physical deformity. As joint damage can proceed rapidly after the onset of symptoms, it is imperative that assessing clinicians have a high index of suspicion in patients with psoriasis presenting with joint and/or musculoskeletal complaints.
Early referral to a rheumatologist is the first initial step in treating PsA. This allows prompt diagnosis and initiation of disease-modifying medication that can arrest the pathological inflammatory process in the joints. In recent years, two specific treatment targets have been identified to maximise patients’ prognosis.
Remission is the ideal therapeutic target, loosely defined as the absence of clinical and serological inflammatory activity, while ‘minimal disease activity’ (MDA) is felt to be an acceptable alternative to remission.
The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) also asserts that all facets of PsA be targeted by treatment, including psoriasis, dactylitis, enthesitis, axial disease (eg, sacroiliitis) and nail involvement.
Treatment of PsA has advanced considerably in the last two decades. With the advent of the tumour necrosis factor inhibitor (TNFi) drugs for RA, clinical trials examining their efficacy in PsA were undertaken, with positive outcomes.
Over the intervening period, a variety of medications with specific immunopathological targets other than TNF have been developed for use in psoriatic disease (psoriasis and/or PsA). Examples include ustekinumab (targeting propagation of the IL-12/IL-23 signal) and secukinumab (IL-17 blockade). Traditional disease-modifying anti-rheumatic drugs (DMARDs) as used in RA are also employed in treating PsA.
‘Disease modification’ in rheumatic disease refers to the ability of a therapy to arrest the pathological process of joint inflammation and, thereby, destruction of the articular structures. These DMARDs include methotrexate (MTX) and leflunomide; however, their role as true modifiers of the disease process in PsA is controversial, in contrast to that of RA.
The PsA therapeutics armamentarium is becoming increasingly complex as the immunopathology of psoriatic disease becomes unravelled. In the paragraphs that follow, a brief discussion relating to each of the individual medications is provided, followed by an outline of their place in the PsA treatment algorithm. It is important to state that in modern practice, consideration be given to patient preference in relation to specific treatments.
<h3 class=”subheadMIstyles”>Initial medications</h3> <p class=”subhead2MIstyles”><strong>NSAIDs</strong>
The non-steroidal anti-inflammatory drugs (NSAIDs) continue to have a role to play in the treatment of PsA, particularly as an initial medication on first presentation to the primary care provider. While there are a variety of NSAIDs to choose from, their efficacy in PsA is felt to be equivalent. While not disease-modifying, the introduction of an NSAID will reduce pain and stiffness for many, and is particularly effective in alleviating the stiffness associated with sacroiliitis and other sites of spinal involvement in PsA. Unless there are contraindications to prescription of an NSAID, they should be considered in patients presenting for the first time. The rheumatologist will then decide whether ongoing prescription is appropriate.
<strong>Corticosteroids </strong>
While prescription of systemic corticosteroid medication for peripheral arthritis in PsA is not uncommon, it should be done with caution due to the potential risk of exacerbation of psoriasis, including provoking the onset of erythroderma. However, in those patients with a monoarthritis or dactylitis of one or two digits, corticosteroid injection can be considered and is often helpful.
<h3 class=”subheadMIstyles”>Oral DMARDs</h3> <p class=”subhead2MIstyles”><strong>MTX</strong>
MTX is the first DMARD most commonly prescribed by rheumatologists for PsA. It has a number of advantages over other medications, including its longevity, established safety profile, low cost to the patient, once-weekly administration, availability in both oral and parenteral form, and therapeutic effect on both joints and psoriasis. There remains, however, a lack of convincing clinical trial data of any true disease-modifying effect attributable to MTX in PsA, although a number of cohort studies have reported positive outcomes with its use. MTX has no effect on PsA-related axial inflammation, while its effect on enthesitis is limited. Onset of action is also quite slow, and can take between eight and 12 weeks for benefits to be seen. Nonetheless, it has a role to play in most patients with moderate disease activity. Patients are advised to abstain or limit alcohol intake when prescribed the drug and in male or female patients planning pregnancy, a ‘washout’ period of between three and six months is recommended due to the risk of teratogenicity. A blood monitoring schedule should also be provided to patients taking MTX, with regular review of liver function and full blood count.
<strong>Leflunomide </strong>
Of the oral ‘traditional’ DMARDs, leflunomide has the strongest evidence for use in PsA. It is taken as a single daily dose with a similar side-effect profile to MTX. Blood monitoring is also required. In women of child-bearing potential considering pregnancy, it may not be the best choice, as it requires a prolonged ‘washout’ period of up to two years. It has a lesser effect on psoriasis compared with MTX and, as with the other traditional DMARDs, has no effect on PsA-related axial disease.
<strong>Sulphasalazine </strong>
Although often prescribed, sulphasalazine has lost favour as a treatment in PsA and has minimal effect on psoriasis. Clinical trial data has been unconvincing in terms of disease modification and its benefit seems to be more in relation to pain control.
It is also cumbersome to take, usually requiring twice-daily intake of two or three large pills. Early discontinuation of the drug is common due to adverse effects, such as gastrointestinal intolerance and rash.
<strong>Apremilast </strong>
Apremilast is a relatively new oral medication targeting phosphodiesterase 4 (PDE4), an enzyme ‘upstream’ in the psoriatic inflammatory pathway. Blockade of this enzyme has resultant downstream effects, including on TNFα and IL-17 expression, key pro-inflammatory cytokines in psoriatic disease. It is taken twice daily. It has proven efficacy in both psoriasis and in symptom control in PsA; however, it has not yet been shown if it can retard or prevent radiographic progression. For that reason, prescription in routine rheumatology practice has been somewhat slow, as clinicians attempt to establish a more precise role for the drug in treating PsA. It may suit some patients who have milder disease and have no evidence of erosion or clinical damage at baseline. It may also be useful in patients with dactylitis or enthesitis-dominant disease, as clinical trial data is positive in that regard. Furthermore, routine blood monitoring and alcohol abstinence are not a necessity. Adverse effects include nausea and diarrhoea for a short period after introduction of the drug. Less commonly, unexplained weight loss has been reported.
<h3 class=”subheadMIstyles”>Biological DMARDs</h3> <p class=”subhead2MIstyles”><strong>TNFi</strong>
There are a number of TNFi medications currently in use for psoriatic disease. These include etanercept, adalimumab, golimumab and certolizumab pegol. While infliximab also has proven efficacy in PsA, unlike the aforementioned drugs, which are self-administered via subcutaneous injection, infliximab is administered by intravenous infusion, thus being less ‘patient-friendly’ and less commonly prescribed. TNFi medications are truly disease-modifying, with clinical trial data consistently reporting delay in joint damage progression. They are also effective against all facets of psoriatic disease, including nail and cutaneous psoriasis, axial disease, enthesitis and dactylitis. Other advantages include a less rigorous schedule of administration for patients, being prescribed as weekly, fortnightly or monthly, depending on the individual drug being taken.
Adverse effects can occur but gastrointestinal intolerance, as seen with the oral DMARDs, is very uncommon with TNFi. Localised cutaneous reaction at the site of injection occurs in half of patients with initial use of these drugs, but tends to lessen with greater use. Leukopaenia is also seen and many rheumatologists choose to monitor a full blood count at regular intervals. There is a greater risk of infection with TNFi use and patients are screened for latent tuberculosis and hepatitis B and C prior to prescription. Vaccination against influenza and pneumococcus is recommended.
TNFi is frequently co-prescribed with an oral DMARD, most commonly MTX. While evidence exists for greater drug survival with TNFi and MTX co-prescription, an additive effect of the combination is generally not seen, as can be seen with RA. Another major disadvantage to use of TNFi is the high cost of the medications to the patient and the State healthcare provider. For that reason, their use as first-line treatment ahead of MTX has not become routine. Despite that, the 2015 GRAPPA treatment recommendations acknowledge that TNFi is appropriate as first-line medication in more severe PsA, particularly in those with many symptomatic joints and in those with early and established radiographic damage. So-called ‘biosimilar’ TNFi drugs have been coming to clinical practice over the last five years. It is hoped that these biosimilar medications will significantly reduce the cost of prescribing TNFi.
<strong>Secukinumab </strong>
IL-17 is a pro-inflammatory cytokine particularly abundant in both psoriatic skin lesions and the synovium of those with PsA. Secukinumab is an IL-17 blocker and clinical trial data has been extremely impressive in those with severe psoriasis, with many patients achieving complete clearance of their disease after weeks to months of treatment. It is also effective in treating PsA in both peripheral and axial joints, also with disease-modifying effects. Secukinumab is prescribed as a self-administered subcutaneous injection, and after once-weekly injections over five weeks, it is taken on a monthly basis. As with TNFi, patients are screened for latent TB and patients are also at greater risk of infection when compared with placebo.
<p class=”subhead2MIstyles”><strong>Ustekinumab</strong>
Ustekinumab is a monoclonal antibody against the p40 subunit that is shared by both IL-12 and IL-23, two cytokines with immunopathological roles in psoriatic disease. Clinical trial data suggests that its effect on joint disease does not quite match that of TNFi and this experience is borne out in daily clinical practice; however, it has a disease-modifying effect and has a role to play, particularly in patients where psoriasis is more problematic than their arthritis.
<h3 class=”subheadMIstyles”>Other considerations</h3>
Increasingly, evidence of an elevated risk of cardiovascular disease in PsA is being presented. All clinicians involved in caring for patients with psoriatic disease should be encouraged to routinely monitor and treat established risk factors for cardiovascular disease, including hyperlipidaemia, obesity and hypertension.
In the last decade, rheumatologists have had their first chance to investigate the state of remission that many patients now achieve, through a combination of more efficacious medication and a more aggressive approach to treating those with recent-onset PsA. One of the next challenges for the specialty of rheumatology will be to identify the best way to lead patients into a sustained drug-free remission. Preliminary studies suggest that a gradual tapering of DMARD medication over months and years has a higher likelihood of success than sudden discontinuation of treatment. The duration of remission while on full-dose medication may also influence outcomes.
The development of new drugs for treatment of PsA has revolutionised rheumatology practice. There remains, however, an ongoing necessity to educate and inform the non-rheumatology clinician about the importance of early diagnosis and treatment of PsA. Greater application of targeted systemic agents (eg, apremilast) in those with psoriasis only (ie, pre-arthritic) could lead to a fall in incidence of PsA over time, while discovery of biomarkers that might identify patients with psoriasis at risk of developing PsA may offer another pathway for treatment development.
<h3>Conclusion</h3>
Psoriatic disease is prevalent and clinicians need to have an index of suspicion for PsA in those with psoriasis complaining of musculoskeletal symptoms. Referral to a rheumatologist to facilitate early diagnosis and institution of a treatment strategy capable of attaining a state of remission within three-to-six months is important to limit and prevent irreversible joint damage and disability.
<p class=”referencesonrequestMIstyles”><em><strong>References on request</strong></em>





Leave a Reply
You must be logged in to post a comment.