Prof Dermot McCaffrey Associate Professor and Consultant Cardiologist and Heart Failure Specialist, Beacon Hospital, Dublin
Heart failure (HF) is a condition associated with ageing, so will become more prevalent in the decades to come as our life expectancy increases. HF is associated with many comorbidities which are also becoming more prevalent, namely hypertension, diabetes, obesity and renal failure.
Definition
HF is a clinical syndrome characterised by typical symptoms that may be accompanied by signs caused by a structural or functional cardiac abnormality resulting in reduced cardiac output and/or elevated intracardiac pressures at rest or during stress. However, a simpler definition is that HF is a clinical syndrome where the heart is unable to meet the body’s normal requirements.
Terminology
Discussing HF can be confusing, as it is defined by aetiology, ie, ischaemic, valvular or hypertensive and by left ventricular ejection fraction (LVEF), where preserved systolic (HFpEF) is if the LVEF is >50 per cent, mid-range (HFmrEF) is if the LVEF is 40-to-49 per cent, or reduced (HFrEF) when LVEF is <40 per cent. HFpEF is now more prevalent than HFrEF, both due to the ageing population and with better recognition of the problem.
An older lady who is breathless walking to the shops would have been told that this was ‘age-related’ or ‘deconditioning’, however, if her GP now investigates her and her ECHO shows LVEF 65 per cent, elevated BNP and ECHO evidence of impaired relaxation with a dilated left atrium, then she has confirmed HFpEF, functional class New York Heart Association (NYHA) II.
She is now a statistic and her one-year mortality is estimated at 7 per cent and risk of hospitalisation at 32 per cent. If she is admitted with acute decompensated heart failure, then her one-year mortality is 17 per cent and risk of rehospitalisation is 44 per cent (European Society of Cardiology (ESC)-HF pilot study). This high hospitalisation rate results in major healthcare and personal costs.
Epidemiology
The prevalence of HF is 1-to-2 per cent of the adult population in developed countries, rising to 10 per cent in those over 70 years. Of those presenting to their GPs with breathlessness, one-in-six will have unrecognised HF. Temporal trends suggest HFrEF is reducing, whereas the prevalence of HFpEF is increasing.
Diagnosing HF
Patients presenting with dyspnoea or fatigue, where HF is a diagnostic possibility after history and examination, should have an ECG, CXR, biomarker test, ie, BNP/NTproBNP, and then proceed to an ECHO to determine their ejection fraction, as the management of HFrEF is quite different to HFpEF. Individual patients may require more specific tests, such as coronary angiography, cardiac MRI looking for fibrosis, and amyloid or hereditary cardiomyopathy such as HCM, etc. In specialist HF clinics, six-minute walk tests, right-heart studies, myocardial biopsy, PET scans, and genetic testing may all be considered. The cause of the patient’s HF is important to identify, as there are some reversible conditions, such as alcoholic, chemotherapy-related or thyrotoxic cardiomyopathies, as well as cardiomyopathies associated with tachyarrhythmia, ie, fast AF or malignant hypertension.
Management
The aim of HF management is to improve symptoms, keep the patient out of hospital and prolong survival. These aims may vary, depending on an individual patient’s circumstance, ie, at extremes of age where symptom control may be more pertinent then longevity.
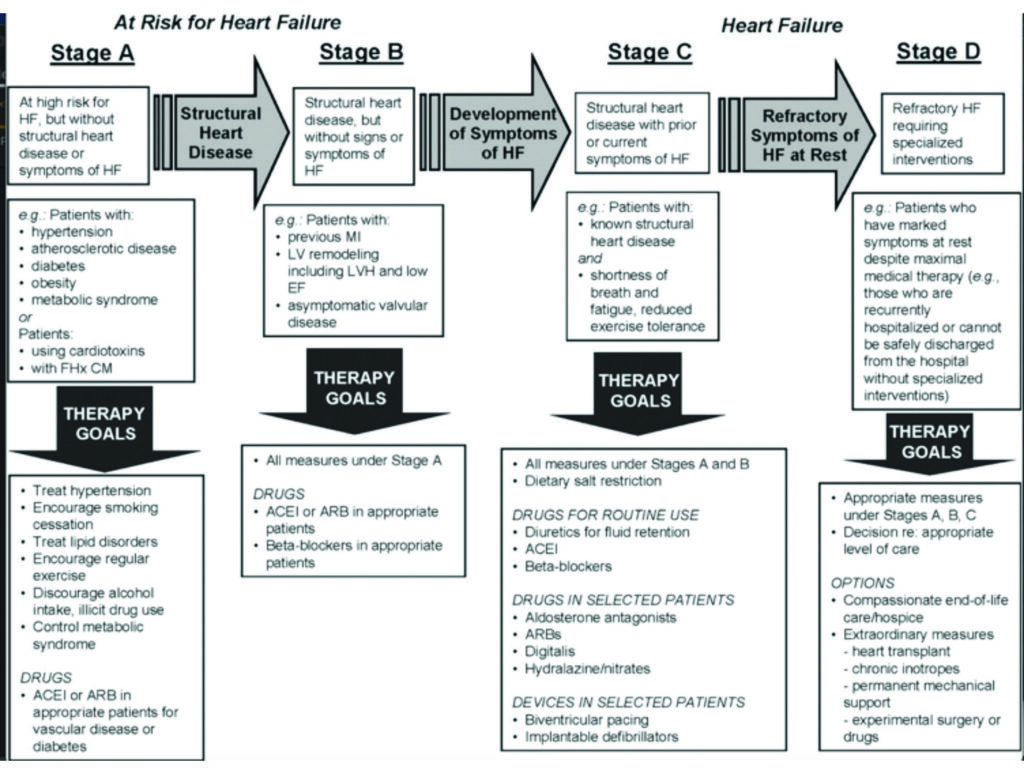
Management of HF should emphasise prevention. With this in mind, the American College of Cardiology (ACC) emphasises stages when someone is at risk and then progressing to having symptomatic HF with the intention of encouraging physicians to intervene during the early stages to prevent progression. See Figure 1.
Stage A are those at risk, Stage B patients have some damage to the heart, Stage C patients have symptoms of HF, and finally, Stage D are severely symptomatic and need specialised support (Stage D patients would be considered NHYA IV/V functional class).
Pyramid of HF
Another way of looking at the burden of HF is the ‘pyramid’ concept, where patients at the top of the pyramid require acute hospital care and thus incur large costs for a small number of people, compared to those at the bottom of the pyramid. Those at the bottom are many and are at risk of HF, but can be managed in the primary care setting. Their primary physicians can identify and treat risks, ie, hypertension, diabetes, etc, thus preventing them from ever getting to the top of the pyramid and needing acute care.
Drug treatment of HF
HFpEF: There are no proven therapies that reduce the morbidity and mortality of HFpEF. The management therefore is to treat associated comorbidities, especially hypertension and diabetes (preferably with ACEi/ARB but consider aldactone (TOPCAT sub-study)). Also, improving the filling time by slowing the heart rate with either beta blockers or verapamil may assist with breathlessness. HFpEF patients benefit from judicious use of diuretics but with close observation of their renal function, as they tolerate hypovolaemia poorly.
HFrEF: Those with impaired LVEF have a higher morbidity and mortality rate than HFpEF but there are many proven therapeutic options. The remainder of this piece therefore focuses on HFrEF, with particular emphasis on two of the newer HF drugs available since 2017.
(1) Diuretics improve symptoms but not morbidity or mortality. (2) ACEi reduce mortality by 16-to-20 per cent. (3) Beta blockers reduce mortality by 30-to-35 per cent. (4) ARBs reduce mortality by 10 per cent. (5) Aldosterone antagonists improve mortality by 25 per cent. Two new HF therapies that have reduced morbidity and mortality are: (6) ARNIs reduce morality by 20 per cent; and (7) SGLT2 inhibitors reduce mortality by a further 20 per cent. I discuss these two new agents below in greater detail.
Devices are not covered in this piece but are pivotal in management of HFrEF patients with low LVEF, reducing mortality (ICD) and improving symptoms (CRT-D).
Angiotensin receptor blocker and neprilysin inhibition (ARNI)
ARBs have been shown to be equivalent in HFrEF in those patients intolerant to ACEi (CHARM Preserved)2. ARBs reduce vasoconstriction, sodium and water retention and myocardial hypertrophy. The neprilysin inhibitor sacubitril prevents the breakdown of vasoactive peptides (natriuretic peptides (ANP/BNP), adrenomedullin, bradykinin, substance P, calcitonin gene-related peptide). The main natriuretic peptide, BNP, binds to NP receptors and augments generation of cGMP, thereby enhancing diuresis, natriuresis and myocardial relaxation and Inman-remodelling.
BNP is produced by the ventricular endothelium in response to stretch/strain and the PARADIGM-HF trial1 showed that augmenting the body’s own defence mechanism confers significant benefit in morbidity and mortality.
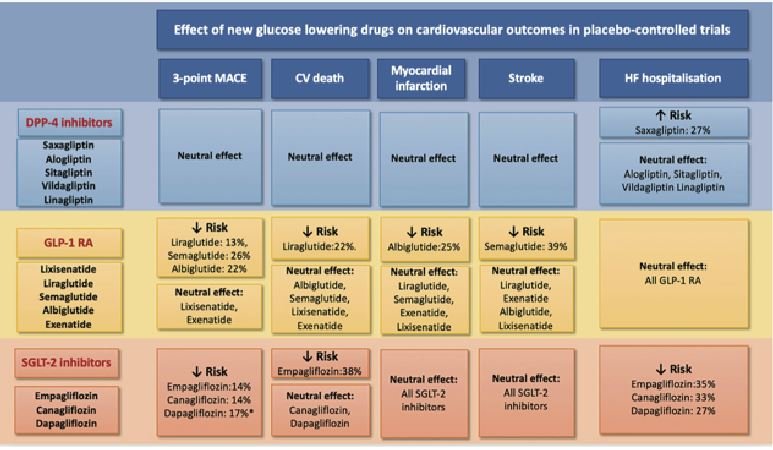
(Images from the recent ESC/HFA position paper on glucose-lowering drugs in patients with heart failure)
The ARB valsartan and neprilysin inhibition (NI) sacubitril was shown in PARADIGM-HF to be superior to enalapril (ACEi) in reducing CV death and HF hospitalisation by 20 per cent, but also sudden cardiovascular death (SCD) by 20 per cent and all-cause mortality by 15 per cent, as well as improving symptoms. In Ireland, patients currently need to see an approved prescriber who can identify those who meet certain criteria for Entresto (valsartan+sacubitril). Of note, patients on Entresto were on lower diuretic doses at the end of the trial. Diuretics cause RAAS activation, which is deleterious to HFrEF, so perhaps less diuretic is a good target for our HF patients? This lower diuretic dose was also observed in the SGLT-2 inhibitor study DAPA-HF.4
Issues with prescribing Entresto: When changing from an ACEi to Entresto, there must be a 36-hour ACEi-free period to avoid a small risk of angioedema. Patients need a U&E checked a month post Entresto initiation and expect a small rise in creatinine, which does not reflect a cardiorenal problem. Entresto has three doses, namely 24/26mg, 49/51mg, and 97/103mg twice-daily. One should attempt to up-titrate to 97mg (sacubitril) and 103mg (valsartan) bd. Note that 206mg of valsartan is a higher daily dose then usually used, either in HF or hypertension, but this dose was well tolerated in PARADIGM-HF. This observation should encourage us to up-titrate ACEi to high doses in HFrEF patients, even if clinically stable. (ATLAS Study Group, Comparison of low and high doses of ACEi in chronic heart failure).6
The following patients should be considered for Entresto: Those with HFrEF who are symptomatic NYHA II, III and IV with LVEF <35 per cent, preferably on optimal HF therapy including BB, ACEi/ARB and aldosterone antagonists, systolic BP >100mmHg and K+ <5.4mmol/L. The HSE notes that the annual cost of Entresto is €1,807, compared to €51 for ramipril 10mg or €118 for candesartan 32mg.
Replacing an older drug with a new drug that adds benefit by reducing both HF hospitalisation and death by 20 per cent is usually well received
One of the attractions of Entresto is that it is a ‘replacement’ for an ACEi/ARB, rather than an additional tablet. HFrEF patients are already taking a beta-blocker, diuretic and aldosterone antagonist. However, replacing an older drug with a new drug that adds benefit by reducing both HF hospitalisation and death by 20 per cent is usually well received. Also, within three months, patients self-report feeling better, which is encouraging. This functional response is seen in both chronic HFrEF patients and those with a more recent diagnosis.
Sodium-glucose cotransporter 2 (SGLT-2) inhibitors: HF is present in 28 per cent of diabetics and of those diabetics with HF, 25 per cent have HFrEF and 75 per cent have HFpEF. Some non-insulin dependent diabetes mellitis (NIDDM) drugs such as the DPP-4 inhibitor saxagliptin should be avoided in HF patients. However, the GLP-1 receptor agonist liraglutide has a neutral effect on HF. The SGLT2 inhibitors were seen to have a significant reduction in HF hospitalisation and in CV death as a secondary outcome. EMPA-REG outcomes trial3 of empagliflozin in NIDDM patients with established cardiovascular disease showed a 38 per cent reduction in CV mortality and 35 per cent reduction in HF hospitalisation. Interestingly, it also showed less diuretic use in the SGLT-2 inhibitor arm (as was noted in the PARADIGM-HF trial). The other two SGLT-2 inhibitors, canagliflozin and dapagliflozin, also showed benefit in HF hospitalisation and cardiovascular events and dapagliflozin showed benefit in non-diabetic HF patients too.
Mechanism of action: SGLT-2 inhibitors reduce glycaemia by promoting glycosuria, so have a mild diuretic effect as water is lost by the pull of the oncotic glucose. However, this was insufficient to explain the reduction in HF hospitalisations. SGLT-2 inhibitors were also the first diabetic drug class to show a mortality benefit which was independent of its effect on HbA1C.
Possible mechanisms on how SGLT-2 inhibitors reduce heart failure include: (1) Reduction in preload and afterload; (2) alternative energy supply, ie, ketones,such as beta-hydroxybutyrate; (3) direct inhibition of Na+/H exchange in the myocardium; (4) reduction in LV mass and improved diastolic function; (5) improved endothelial dysfunction; and (6) stimulation of glucagon secretion, which reduces peripheral vascular resistance.
Because of these encouraging HF benefits in patients with NIDDM, the DAPA-HF study4 looked at dapagliflozin 10mg in 4,744 HFrEF patients, both with and without NIDDM. This study showed a 38 per cent reduction in worsening HF events and a 27 per cent reduction in CV death/HF hospitalisation/urgent HF visit end-point in the non-diabetic population (n=2,605).
There was no increased hypotension or renal issues but there were more genital infections, as expected in an agent that promotes glucosuria, which can be managed with antifungals such as Canesten and good hygiene. We therefore now have a fifth agent for HFrEF for those with LVEF <40 per cent, irrespective of whether they have NIDDM or not.
The question in 2020 will be which HFrEF patient is likely to benefit from an SGLT-2 inhibitor. Where does SGLT-2 inhibition fit in the ESC therapeutic algorithm for HFrEF? One would assume that initially, dapagliflozin 10mg should be prescribed in HFrEF patients who also have NIDDM and are felt to be at risk of HF events.
In summary
In summary, HF is a prevalent condition which, if recognised early, can be treated effectively with significant improvement in cardiovascular outcomes. HFrEF patients with persistently low LVEF <35 per cent should be considered for ARNI treatment and those who are already on optimal pharmacotherapy with type 2 diabetes should be considered for SGLT-2 inhibitors that have shown significant benefit in reducing HF hospitalisations and CV death.
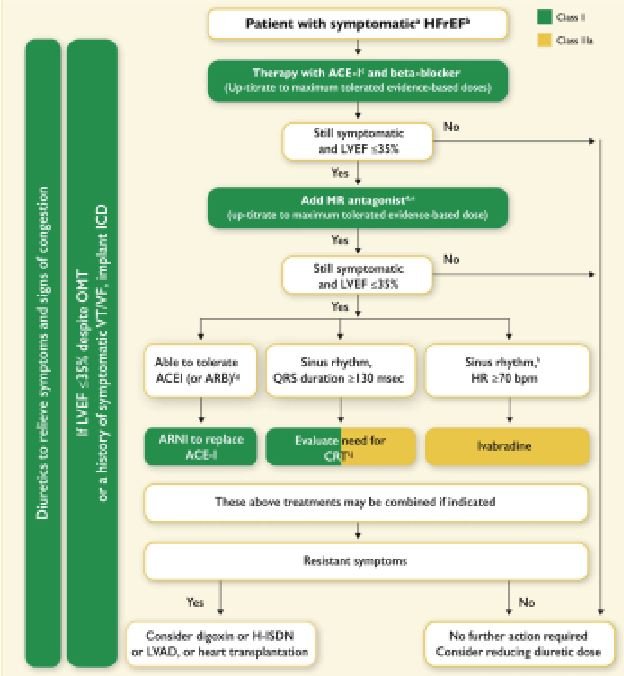
HFrEF patients still receive a stepwise introduction of treatments (Figure 2) and each treatment should be up-titrated to their optimal dose.
This may need expert guidance by a HF specialist in certain HFrEF patients who have been identified as being at risk of an adverse outcome, often due to comorbidities such as COPD, chronic renal failure, etc.
Better public awareness of HF signs and symptoms should result in patients presenting to their primary physicians earlier. This will allow earlier diagnosis, treatment and better outcomes using the therapies outlined above.
However, these patients would already be classed as Stage C by the ACC, whereas we should preferably target patients who are in Stage A, where primary physicians can intervene and prevent progression to HF.
HF disease management programmes (DMP) for those with established HF have been proven to result in better patient outcomes and less HF hospitalisation. Patient support groups allow patients and their carers to share their experiences and learn tools, such as how to avoid dietary indiscretion, ie, high salt loads, etc, and improve drug compliance, which avoids HF decompensation episodes, and encourage HF exercise programmes to avoid associated musculoskeletal problems.
Patients can share and learn from their experiences and also educate physicians on their individual experience with HF.
A not-too-simple task that I ask my HF patients is that they are able to tell me what each drug is doing to help their heart. “That one makes me wee,” “that one slows my heart”, “that one prevents scarring” and “that one reduces the pressure in my arteries and has been shown to make me live longer” – this degree of knowledge improves compliance greatly.
‘With knowledge comes power’ and as HF is a complex condition, there is a lot of knowledge that patients need to understand to optimally look after themselves, which can be overwhelming.
Therefore, the more information that we as healthcare providers can impart in a clear and precise way, the better the patient can manage their own condition.
Comments to dermot.mccaffrey@beaconhospital.ie.
References
- Prospective Comparison of ARNI with ACEi to Determine Impact on Global Morbidity and Mortality in Heart Failure, NEJM 2015.
- CHARM — Alternative trial. Lancet 2003:362.
- Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. EMPA-Reg OUTCOME. NEJM 2015; 373.
- Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction: DAPA-HF Trial: NEJM 2019: 381.
- ESC/HFA position paper on the role and safety of new glucose-lowering drugs in patients with heart failure. EJHF Dec 2019
- ATLAS study group: Circulation 1999: 100.










Leave a Reply
You must be logged in to post a comment.