S KELLIHER, E O’ROURKE, SHANE P COMER, PAULINA B SZKLANNA, LUISA WEISS, PB MAGUIRE, F NÍ ÁINLE, and B KEVANE
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is the causative pathogen associated with the novel human viral illness, Coronavirus disease 2019 (Covid-19). This disease was first described in Wuhan, China in December 2019 and to date over 68 million people worldwide have been infected. Current data indicates that the death toll associated with this disease now stands at over 1.56 million individuals.
Although Covid-19 is predominantly an illness affecting the respiratory system, a distinct coagulopathy characterised by a thrombotic tendency with potentially devastating complications has emerged in association with this disease. In the midst of the ongoing global pandemic, and with the prospect of a second wave of infections facing many countries in the coming months, there is an urgent need to understand the molecular mechanisms underlying the coagulopathy associated with Covid-19 in order to optimise preventative and therapeutic strategies.
The crosstalk between inflammation and blood coagulation – relevance in Covid-19?
A complex interplay is known to exist between the activity of inflammatory pathways and blood coagulation activation and this interplay has been shown to represent a source of morbidity in a range of systemic diseases including acute respiratory distress syndrome, inflammatory bowel disease, cancer metastasis and pulmonary vascular diseases.
Cells of the innate immune system, including neutrophils and monocytes, can express procoagulant substances on their cell surface in response to certain pro-inflammatory stimuli (including inflammatory cytokines and invading pathogens), a phenomenon which can lead to dysregulated intra-vascular coagulation activation and fibrin formation.
Interestingly, this interplay between coagulation and inflammation is not a one-way process, and products of coagulation activation such as thrombin and other coagulation proteases can also mediate pro-inflammatory effects by signalling on immune and vascular cells, modulating the activity of inflammatory pathways and leading to the activation of pro-inflammatory intra-cellular signalling in endothelial cells and leucocytes.
Severe Covid-19 is a pro-inflammatory state characterised by fever, elevated inflammatory markers and pneumonitis. Even at the earliest stages of the pandemic, data emerging from China and other regions suggested that derangements of blood coagulation were common among infected individuals. Moreover, levels of these markers of coagulation activation (such as elevated D-dimer) appeared to correlate with disease severity and mortality risk.
The activity of the inflammatory-haemostatic axis in Covid-19 appears to generate a unique laboratory profile in contrast to other pro-inflammatory infective states. Elevated D-dimer, prolonged prothrombin time and thrombocytopaenia appear to be the most frequently observed coagulation derangements.
In contrast, prolongation in the activated partial thromboplastin time, reduction in fibrinogen levels and overt DIC (by ISTH Criteria) appear to occur relatively less frequently in Covid-19 in comparison to other critical illnesses associated with coagulopathy.
The molecular mechanisms underlying this unique coagulopathy remain to be fully characterised, however, determining the nature of this interplay would likely be of major clinical relevance in light of the significant clinical challenges posed by abnormal coagulation activation in this disease.
Thrombotic complications associated with Covid-19
Anecdotal reports suggesting that high rates of venous thromboembolism (VTE) among Covid-19 affected individuals were present at an early stage of the pandemic were subsequently supported by data from Chinese cohort studies which reported rates of VTE as high as 25 per cent among sub-groups of affected patients.
However, rates of hospital-acquired VTE and the approach to VTE prevention in hospitalised patients is somewhat different in China and other Asian populations where widespread use of pharmacological thromboprophylaxis is not always routine (in contrast to Western countries) and so these initial data were interpreted with caution in other parts of the world (particularly in Europe and North America).
However, the first European data which emerged from the Netherlands in April 2020 closely mirrored the rates of VTE detected in these initial Asian reports. In this first European study, the investigators (FA Klok et al, Thrombosis Research 2020) reported a cumulative incidence of VTE of 31 per cent in a cohort of 184 Covid-19 ICU patients. Crucially, all of these patients had been receiving low molecular weight heparin (LMWH) thromboprophylaxis.
Moreover, symptomatic VTE made up the majority of these thrombotic events and included 18 patients who developed pulmonary emboli that were at least at the segmental level. Subsequent data from another Dutch centre (S Middelorp et al, Journal of Thrombosis and Haemostasis 2020) reported a 14-day cumulative incidence of symptomatic VTE of 31 per cent among Covid-19 patients managed in the ICU (all of whom had been receiving LMWH thromboprophylaxis).
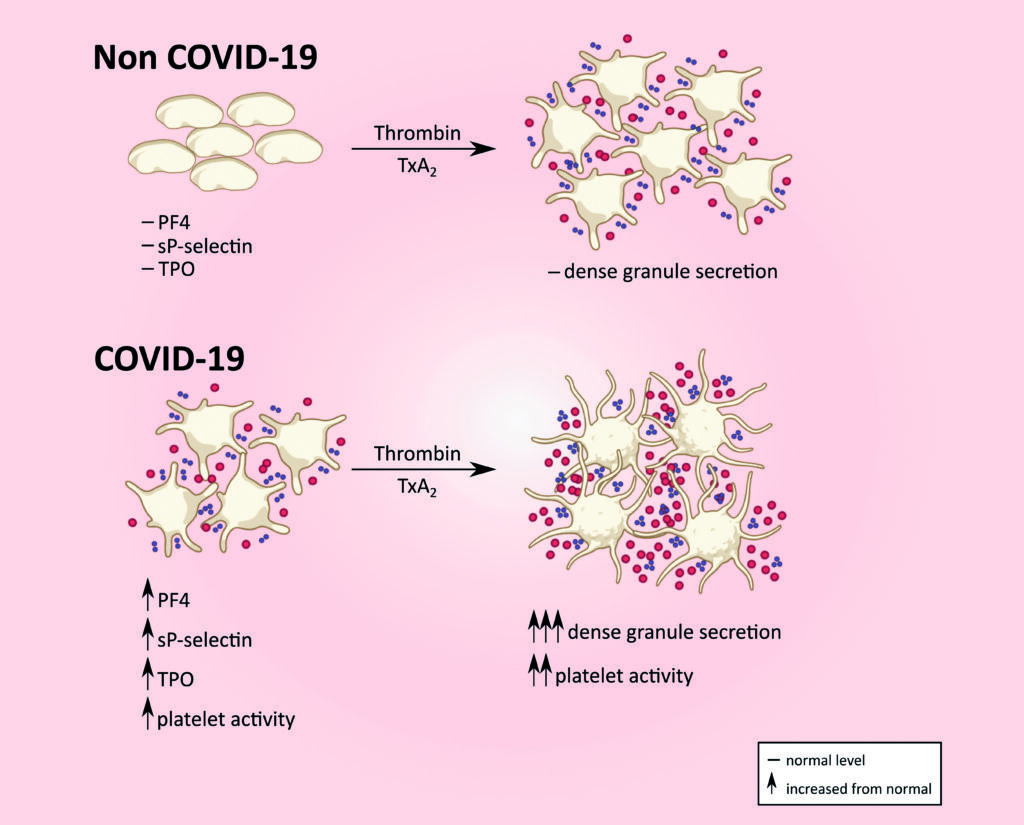
Levels of platelet activation markers such as sP-selectin, PF4 and TPO are elevated in Covid-19 in contrast to that seen in unaffected individuals. Platelet dense granule secretion in response to thrombin/thromboxane A2 is also enhanced in Covid-19. (soluble P-selectin, sP-selectin; platelet factor 4, PF4; thrombopoeitin, TPO)
The rate of symptomatic VTE among patients with less severe disease who did not require ICU level care was 3.2 per cent. A number of subsequent studies from centres in France and Italy generated similar findings, reporting cumulative incidence rates of thrombotic complications associated with Covid-19 in the range of 20.4-43 per cent.
More recently, two large retrospective studies from the US have reported rates of thrombotic complications among critically ill Covid-19 patients which are somewhat lower than those reported in Europe (9.3-18.1 per cent), but which confirm the clear distinction observed in most reports to date, where the rates of VTE among patients requiring ICU level care are significantly greater than expected, despite LMWH thromboprophylaxis.
The interpretation of the results of the studies to date is limited by several factors including the retrospective nature of these studies, the relatively small numbers of patients included in individual studies, the differences in use of pharmacological thromboprophylaxis between centres and, crucially, the marked heterogeneity in the statistical methodologies employed by the various investigators in estimating and reporting VTE incidence in their study populations.
Notwithstanding these potential limitations, the apparent high-rates of VTE occurring despite standard pharmacological thromboprophylaxis in patients with severe Covid-19 has become a major source of concern for clinicians involved in the care of these patients. However, due to the current lack of prospective, randomised trial data in this area, the true magnitude of VTE risk in this population and the optimal approach to prevention remains to be determined.
Escalated LMWH dosing strategies have been suggested for high-risk Covid-19 patients. However, data from a multi-centre retrospective US cohort (Al-Samkari et al; Blood, July 2020) suggests that bleeding risk is also significant among Covid-19 patients with critical illness. Current international consensus guidelines provide somewhat conflicting advice in this regard, reflecting the paucity of high-quality data to guide decision-making in this high-risk scenario.
The American College of Chest Physicians (ACCP) CHEST Guideline and Expert Panel Report on the prevention, diagnosis and treatment of VTE in patients with Covid-19 suggest that all hospitalised patients with Covid-19 receive pharmacological thromboprophylaxis (eg, LMWH), but recommend using standard thromboprophylaxis regimens in favour of escalated dosing even among patients with critical illness, in recognition of the lack of evidence confirming the safety and efficacy of an escalated dosing strategy.
In contrast, a clinical guidance document published by the International Society on Thrombosis and Haemostasis Scientific and Standardisation Committee (ISTH SCC) has suggested that intermediate LMWH dosing (eg, twice-daily LMWH thromboprophylaxis) may be considered among high-risk patients (ie, Covid-19 patients requiring ICU management).
Randomised studies are clearly urgently needed to clarify the optimal approach to VTE prevention in Covid-19. The RAPID Trial (ClinicalTrials.gov Identifier: NCT04362085) is an international, multi-centre randomised controlled trial which is comparing the safety and efficacy of standard versus therapeutic-intensity LMWH in the prevention of VTE in patients with Covid-19 who meet specific criteria which indicate the presence of significant coagulopathy (based on D-dimer levels).
Irish patients have had the opportunity to participate in this clinical trial. Collaborating with international lead Dr Michelle Sholzberg, this trial is being led in Ireland by Prof Fionnuala Ní Áinle, Consultant Haematologist at the Mater Misericordiae University Hospital, Dublin, and Co-Director of the Irish Network for VTE Research (INViTE) working with the UCD Clinical Research Centre under the direction of Prof Peter Doran.
It is likely that the clinical approach to VTE prevention and treatment in Covid-19 will continue to evolve pending the completion of high-quality clinical trials, such as the RAPID trial and other related studies. However, there is also currently a significant knowledge gap relating to the molecular mechanisms underlying the unusual coagulopathy associated with Covid-19.
A deeper understanding of these molecular mechanisms would likely provide valuable insights into the pathophysiology of thrombosis in Covid-19 as well as the optimal approach to prevention and treatment.
The aetiology of coagulopathy in Covid-19
The precise mechanisms underlying haemostatic derangements in Covid-19 remain to be fully elucidated. The phenomenon of ‘immunothrombosis’ is likely to be implicated in this process. It has been hypothesised that the inflammatory response, which arises in pulmonary tissues following infection with SARS-CoV-2 activates procoagulant pathway activity leading to thrombosis in the pulmonary micro-vasculature as well as a systemic hypercoagulable state.
Data in support of this hypothesis has been generated in numerous studies which have reported evidence of endothelial inflammation and dysfunction (leading to loss of endogenous anticoagulant activity), markedly enhanced levels of circulating procoagulant factors (such as von Willebrand factor and factor VIII) and reductions in levels of circulating endogenous anticoagulant factors (such as antithrombin) among infected individuals.
The COCOON Study (Covid-19 Coagulopathy and Thrombosis: Novel Prognostic and Therapeutic Opportunities) is an Irish multi-disciplinary clinical and translational study being led by Dr Barry Kevane, Consultant Haematologist at the Mater Hospital and a principal investigator with the UCD Conway SPHERE Research Group.
Data generated to date by the Conway SPHERE Group have demonstrated a distinct pattern of platelet hyper-reactivity among patients with Covid-19, which is most marked among those with severe disease requiring critical care support
This study, which is supported by a Science Foundation Ireland Covid-19 Rapid Response award, aims to characterise the molecular mechanisms underlying coagulopathy in Covid-19, with a particular focus on identifying procoagulant platelet and endothelial dysfunction. Data generated to date by the Conway SPHERE Group have demonstrated a distinct pattern of platelet hyper-reactivity among patients with Covid-19, which is most marked among those with severe disease requiring critical care support.
In particular, they have demonstrated markedly abnormal agonist-induced platelet granule release and platelet activation marker levels among Covid-19 affected patients in contrast to non-Covid-19 affected hospitalised patients and have also reported data indicating distinct patterns in specific routine clinical platelet parameters (including mean platelet volume), which appear to be associated with disease severity in Covid-19.
hese data reflect similar findings of abnormally enhanced platelet activity which have been generated by other research groups in recent months, suggesting that platelets may play a key mechanistic role in Covid-19 related hypercoagulability.
The clinical implications of these initial preliminary data remain to be determined. Work is continuing in the COCOON study with further results expected early in 2021.
Conclusion
The Covid-19 pandemic has generated significant challenges for healthcare providers. These challenges are likely to persist until such time as either the emerging vaccines or effective anti-viral therapies become widely available.
The coagulopathy associated with Covid-19 appears to represent a major source of morbidity among affected individuals. While efforts are ongoing both internationally and here in Ireland to undertake clinical and translational research to further define optimal treatment strategies and elucidate underlying mechanisms, clinicians continue to be faced with high-risk clinical scenarios where decision-making is not yet supported by high-quality data.
Careful assessment of competing haemorrhagic and thrombotic risks, adherence to existing evidence-based guidance and supporting ongoing research in this field will be crucial to improving patient outcomes as we continue to deal with the second wave of SARS-CoV-2 infections over the coming months.










Leave a Reply
You must be logged in to post a comment.