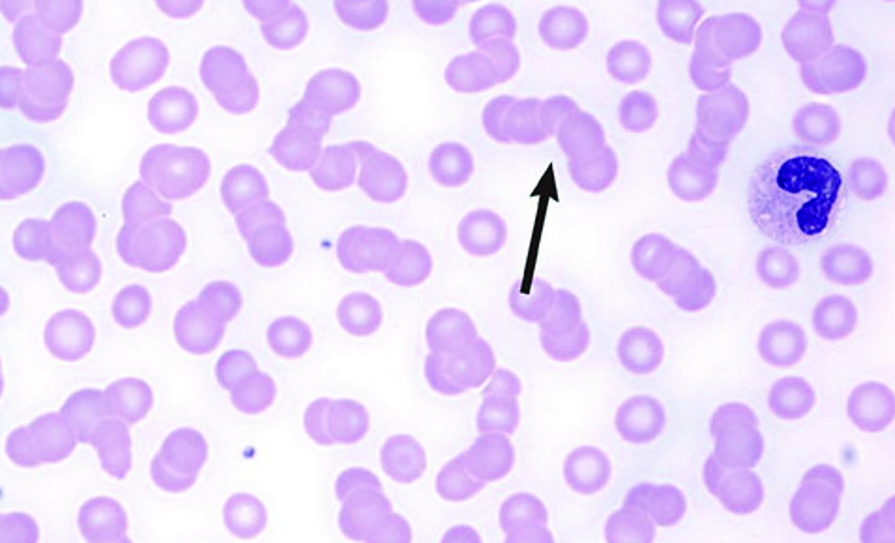
Rouleaux formation
First described in 1848, multiple myeloma (MM), also known as plasma cell myeloma, is characterised by a proliferation of malignant plasma cells and a subsequent overproduction of monoclonal paraprotein (M-protein).
There are several types of plasma cell neoplasms. They include monoclonal gammopathy of undetermined significance (MGUS), isolated plasmacytoma of the bone, extramedullary plasmacytoma, multiple myeloma, and plasma cell leukaemia.
The incidence of MM is two-to-four/100,000. The male-to-female ratio is 1.6:1, and the median age is about 65 years. Prevalence in black people is twice that in white people. A etiology is unknown, although chromosomal and genetic factors, radiation, and chemicals have been suggested.
The average number of new cases per year in Ireland is 305, with approximately 169 deaths annually. The average age at diagnosis is 70 years.
Clinical features
The presentation of MM can range from asymptomatic to severely symptomatic, with complications requiring urgent treatment. Many patients are identified when routine laboratory tests show an elevated total protein level in the blood or anaemia. Systemic complications include bleeding, infection, and renal failure; pathologic fractures and spinal cord compression may occur.
Presenting symptoms of MM include the following:
Bone pain: The most common symptom of myeloma is bone pain. About seven-in-10 people (70 per cent) have lower back or rib pain. The lumbar spine is one of the most common sites of pain.
Pathologic fractures: Pathologic fractures are very common in myeloma. MM causes both generalised bone loss throughout the body as well as areas of bone destruction ‘lytic lesions’ on x-ray in specific areas. The bone loss and bony erosions can lead to osteoporosis and fractures. Many individuals with MM experience fractures of the vertebrae, which can lead to a loss of height; about 30 per cent of individuals experience fractures in other bones, often with little or no preceding trauma. For this reason, they are called ‘pathologic fractures’.
Weakness and malaise: Anaemia, which may be quite severe, is the most common cause of weakness in patients with MM.
Bleeding and anaemia: Occasionally, a patient may come to medical attention for bleeding or anaemia resulting from heavy bone marrow involvement by myeloma. Rarely, monoclonal protein may absorb clotting factors and lead to bleeding.
Infection (often pneumococcal): Abnormal humoral immunity and leukopaenia may lead to infection. Pneumococcal organisms are commonly involved, but shingles (herpes zoster) and Haemophilus infections are also more common among patients with MM.
Hypercalcaemia: Because bones contain large amounts of calcium, the breakdown of bone in MM can lead to hypercalcaemia.
High blood calcium levels occur in 10-to-15 per cent of individuals, and the symptoms may include loss of appetite, nausea, vomiting, frequent urination, increased thirst, constipation, weakness, confusion, stupor, or coma. Symptoms are more common at high calcium blood values (12.0mg/dL or 3mmol/l). Severe hypercalcaemia (above 15-16mg/dL or 3.75-4mmol/l) is considered a medical emergency: At these levels, coma and cardiac arrest can result.
Spinal cord compression: Myeloma can develop in the bones of the spine. Sometimes this can weaken the bone and put pressure on the spinal cord with spinal cord compression (SCC). SCC can cause pain, muscle weakness, and sometimes tingling and numbness of the limbs. If the lower spine is affected, it may also affect how the bowel and bladder work. The symptoms that should alert physicians to consider SCC are back pain, weakness, numbness, or dysaesthesias in the extremities.
Because SCC in MM occurs at multiple levels, comprehensive evaluation of the spine is warranted, including a full spine MRI scan. Patients who are ambulatory at the start of therapy have the best likelihood of preserving function and avoiding paralysis. SCC should be treated urgently with steroids and radiotherapy. Sometimes chemotherapy can be given to help reduce the pressure on the spinal cord. Surgery may be needed to repair or remove the affected bone.
Renal failure: In many patients, renal failure (myeloma kidney) is present at diagnosis or develops during the course of the disorder. In the majority of cases, renal impairment is caused by the accumulation and precipitation of light chains, which form casts in the distal tubules, resulting in renal obstruction. Renal failure in myeloma has many other causes, eg, hypercalcaemia, recurrent renal infections, analgesic-associated nephropathy (NSAIDs), hyperuricaemia, and amyloidosis. Patients also often develop anaemia usually due to kidney disease or suppression of erythropoiesis by cancer cells, but sometimes also due to iron deficiency.
Hyperviscosity: Rarely, myeloma causes a very high level of paraprotein in the blood. This means the blood can become thicker than normal. Normal plasma viscosity is between 1.4 and 1.8 centipoise, while symptoms from hyperviscosity typically occur when plasma viscosity is greater than four centipoise (about four times more viscous than water) and require emergency treatment. Hyperviscosity may be associated with a number of symptoms, including generalised malaise, infection, fever, paresthesia, sluggish mentation, and sensory loss. Patients may report headaches and somnolence, and they may bruise easily and have a hazy vision. Patients with MM typically experience these symptoms when their viscosity is greater than four times that of normal serum. Epistaxis may be a presenting symptom of MM with a high tumour volume. Occasionally, patients may have such a high volume of monoclonal protein that their blood viscosity increases, resulting in complications such as stroke, myocardial ischaemia, or infarction. Plasmapheresis may be used to decrease viscosity.
Neurologic symptoms: Carpal tunnel syndrome is a common complication of myeloma. Meningitis (especially that resulting from pneumococcal or meningococcal infection) is more common in patients with MM. Some peripheral neuropathies have been attributed to MM. Long-term neurologic function is directly related to the rapidity of the diagnosis and the institution of appropriate therapy for MM.
Pathophysiology
The M-protein produced by the malignant plasma cells is IgG in about 55 per cent of myeloma patients and IgA in about 20 per cent; of patients producing either IgG or IgA, 40 per cent also have Bence Jones proteinuria, which is free monoclonal kappa (κ) or lambda (λ) light chains in the urine. In 15-to-20 per cent of patients, plasma cells secrete only Bence Jones protein (light chain myeloma). IgD myeloma accounts for about 1 per cent of cases. Rarely, patients have no M-protein in the blood and urine, although a new serum-free light chain assay now demonstrates monoclonal light chains in many of these patients.
Diffuse osteoporosis or discrete osteolytic lesions develop, usually in the pelvis, spine, ribs, and skull. Lesions are caused by bone replacement by expanding plasmacytomas or by cytokines that are secreted by malignant plasma cells that activate osteoclasts and suppress osteoblasts. The osteolytic lesions are usually multiple; occasionally, they are solitary intramedullary masses. Increased bone loss may also lead to hypercalcaemia. Extramedullary solitary plasmacytomas are unusual, but may occur in any tissue, especially in the upper respiratory tract.
In many patients, renal failure (myeloma kidney) is present at diagnosis or develops during the course of the disorder. Renal failure has many causes, most commonly, it results from deposition of light chains in the distal tubules or hypercalcaemia. Patients also often develop anaemia, usually due to kidney disease or suppression of erythropoiesis by cancer cells, but sometimes also due to iron deficiency.
Susceptibility to bacterial infection may occur in some patients. Viral infections, especially herpes zoster infections, are increasingly occurring as a result of newer treatment modalities, especially the use of the proteasome inhibitors bortezomib and carfilzomib.
It is estimated that 10-to-15 per cent of MM patients will experience symptoms from the development of amyloidosis during the course of their disease. However, as many as 38 per cent of myeloma patients may develop amyloidosis, but experience none of its symptoms.
Amyloidosis is a disease in which proteins accumulate in organs such as the heart, kidney, liver, nerves, or intestines, leading to organ damage.
Diagnosis
MM is suspected in patients >40 years with persistent unexplained bone pain, particularly at night or at rest, other typical symptoms, or unexplained laboratory abnormalities such as elevated blood protein or urinary protein, hypercalcaemia, renal insufficiency, or anaemia.
Anaemia is present in 80 per cent of patients, usually normocytic-normochromic anaemia with formation of rouleaux, which are clusters of three-to-12 RBCs that occur in stacks. White blood cell (WBC) and platelet counts are usually normal. ESR usually is >100mm/h; BUN, serum creatinine, LDH, and serum uric acid may be elevated. Anion gap is sometimes low. Hypercalcaemia is present at diagnosis in about 10 per cent of patients.
Protein electrophoresis is done on a serum sample and on a urine sample concentrated from a 24-h collection to quantify the amount of urinary M-protein. Serum electrophoresis identifies M-protein in about 80-to-90 per cent of patients. The remaining 10-to-20 per cent are usually patients with only free monoclonal light chains (Bence Jones or light chain myeloma) or IgD. They almost
always have M-protein detected by urine protein electrophoresis.
Immunofixation electrophoresis can identify the immunoglobulin class of the M-protein (IgG, IgA, or uncommonly IgD, IgM, or IgE) and can often detect light-chain protein if serum immunoelectrophoresis is falsely negative; immunofixation electrophoresis is done even when the serum test is negative if MM is strongly suspected.
Serum free light-chain analysis with delineation of kappa and lambda ratios helps confirm the diagnosis and can also be used to monitor efficacy of therapy and provide prognostic data.
Serum level of beta-2 microglobulin is measured if diagnosis is confirmed or very likely and, along with serum albumin, is used to stage patients as part of the international staging system for
MM (Table 1).
X-rays include a skeletal survey (plain x-rays of skull, long bones, spine, pelvis, and ribs). Punched-out lytic lesions or diffuse osteoporosis is present in 80 per cent of cases. Radionuclide bone scans usually are not helpful. MRI can provide more detail and is obtained if specific sites of pain or neurologic symptoms are present. PET-CT may provide prognostic information and can help determine whether patients have solitary plasmacytoma or MM.
Bone marrow aspiration and biopsy are done and reveal sheets or clusters of plasma cells; myeloma is diagnosed when >10 per cent of the cells are of this type. However, bone marrow involvement is patchy; therefore, some samples from patients with myeloma may show <10 per cent of plasma cells. Still, the number of plasma cells in bone marrow is rarely normal. Plasma cell morphology does not correlate with the class of immunoglobulin synthesised. Chromosomal studies on bone marrow (eg, using cytogenetic testing methods such as fluorescent in situ hybridisation [FISH] and immunohistochemistry) may reveal specific karyotypic abnormalities in plasma cells associated with differences in survival.
Diagnosis and differentiation from other malignancies (eg, metastatic carcinoma, lymphoma, leukaemia) and monoclonal gammopathy of undetermined significance typically require multiple criteria:
Clonal bone marrow plasma cells or plasmacytoma;
M-protein in plasma and/or urine;
Organ impairment (hypercalcaemia, renal insufficiency, anaemia, or bony lesions).
In patients without serum M-protein, myeloma is indicated by Bence Jones proteinuria >300mg/24-h or abnormal serum-free light chains, osteolytic lesions (without evidence of metastatic cancer or granulomatous disease), and sheets or clusters of plasma cells in the bone marrow.
The 2014 International Myeloma Working Group guidelines for the standard investigative workup in patients with suspected MM include the following studies:
FBC, peripheral blood smear, ESR, and chemistry panel (BUN, creatinine, calcium, uric acid).
Serum and urine assessment for monoclonal protein.
Serum-free light chain assay.
Bone marrow aspiration and/or biopsy.
Serum beta2-microglobulin, albumin, serum immunoglobulins, and lactate dehydrogenase (LDH) measurement.
Standard metaphase cytogenetics.
Fluorescence in situ hybridisation (FISH).
Skeletal survey.
MRI, fluorodeoxyglucose-positron emission tomography (FDG-PET), or low-dose whole-body CT for better detection of bone and extramedullary disease.
Current National Comprehensive Cancer Network (NCCN) clinical practice guidelines on MM also recommend the use of FISH for 1q21 amplification as part of the initial diagnostic workup.
Diagnostic criteria
In the updated 2010 International Myeloma Working Group (IMWG) diagnostic criteria, plasma cell MGUS is defined as having serum M-protein <30g/L, clonal plasma cell population in bone marrow <10 per cent, and absence of end-organ damage (CRAB criteria of MM).
MM is defined as smoldering (asymptomatic) or active (symptomatic).
Criteria for smoldering MM are as follows:
M-protein in serum: IgG ≥30g/L, IgA >10g/L; or
Bence-Jones protein >1g/24-h; and/or
Bone marrow clonal plasma cells ≥10
per cent.
Active MM has been diagnosed using the CRAB criteria (hyperCalcaemia, Renal failure, Anaemia, Bone lesions) for end-organ damage, as follows:
Calcium >11.5mg/dL (>2.65mmol/L);
Creatinine >2mg/dL (177μmol/L or more);
Haemoglobin <10g/dL or 2g/dL <normal;
Lytic or osteopenic bone disease.
In November 2014, however, the IMWG added the following criteria for MM that will require therapy:
Bone marrow plasma cells (BMPCs) ≥60 per cent;
Involved/uninvolved serum free light chain ratio ≥100;
Abnormal MRI with more than one focal lesion, with each lesion >5mm.
The Group noted that these findings have been “associated with near inevitable development of CRAB features in patients who would otherwise be regarded as having smoldering MM”. The presence of any of the CRAB criteria or any of these three additional criteria justifies therapy.
Treatment
Solitary plasmacytomas: These are often treated with radiation therapy. If the plasma cell tumour is not in a bone, it may be removed with surgery. Chemotherapy is only used if MM develops.
Early myeloma: Early myeloma includes smoldering myeloma and stage I disease. Patients with early myeloma can do well for years without treatment.
For many patients, starting treatment early does not seem to help them live longer. These patients are often watched closely without starting chemotherapy or other treatments for myeloma. They may be started on a bisphosphonate (eg, zoledronic acid or pamidronate monthly infusions) if they have bone disease.
Based on how abnormal the plasma cells look under the microscope and the levels of immunoglobulins, some patients with early myeloma have a high risk of progressing to active myeloma and needing treatment. In one study, treating these patients with lenalidomide and dexamethasone, before they developed symptoms or problems, helped them live longer.
Active (symptomatic) myeloma: Patients whose myeloma is stage II or higher or who have light chain amyloidosis are often given drug therapy. The drugs chosen depend on the patient’s health (including their kidney function) and whether a transplant is planned.
Often, a combination containing bortezomib, thalidomide or lenalidomide, and dexamethasone is used. Combinations containing bortezomib are especially helpful in patients with kidney problems and those whose myeloma cells contain certain high-risk chromosome abnormalities.
If the patient is not expected to have a transplant, chemotherapy with melphalan and prednisone (MP) may be used, and can be combined with thalidomide (MPT) or
with Velcade (MPV).
Many drug combinations can be useful in treating myeloma. If a drug stops working (or the myeloma comes back), others can be tried.
Bisphosphonate treatment is often started along with chemotherapy. If the areas of damaged bone continue to cause symptoms, radiation therapy may be used.
Patients with MM also receive supportive treatments such as transfusions to treat low blood cell counts, antibiotics, and sometimes intravenous immunoglobulin (IVIG) for infections.
Immunomodulating agents
The way immunomodulating agents affect the immune system is not entirely clear. Three immunomodulating agents are currently used to treat MM.
The first of these drugs to be developed, thalidomide, caused severe birth defects when taken during pregnancy. Because the other immunomodulating agents are related to thalidomide, there is concern that they could also cause birth defects. That is why all of these drugs can only be obtained through a special programme run by the drug company that makes them.
Because these drugs can increase the risk of serious blood clots, they are often given along with aspirin or a blood thinner.
Side-effects of thalidomide can include drowsiness, fatigue, severe constipation, and painful nerve damage (neuropathy). The neuropathy can be severe, and might not go away after the drug is stopped. There is also an increased risk of serious blood clots that start in the leg and can travel to the lungs.
Lenalidomide is similar to thalidomide. It works well in treating MM. The most common side-effects of lenalidomide are thrombocytopaenia and leukopaenia. It can also cause painful nerve damage. The risk of blood clots is not as high as that seen with thalidomide, but it is still increased.
Pomalidomide is related to thalidomide and is used to treat MM. Some common side-effects include anaemia and leukopaenia. The risk of nerve damage (neuropathy) is not as severe as it is with the other immunomodulating drugs, but it is also linked to an increased risk of blood clots.
Proteasome inhibitors
Proteasome inhibitors work by stopping enzyme complexes (proteasomes) in cells from breaking down proteins important for keeping cell division under control. They appear to affect tumor cells more than normal cells, but they are not without side-effects.
Bortezomib was the first of this type of drug to be approved, and it is often used to treat MM. It may be especially helpful in treating myeloma patients with kidney problems. It is injected into a vein (IV) or under the skin, once or twice a week. Common side-effects of this drug include nausea and vomiting, tiredness, diarrhoea, constipation, fever, decreased appetite, and lowered blood cell counts. The platelet count (which can cause
easier bruising and bleeding) and the WBC count (which can increase the risk of serious infection) are most often affected. Bortezomib can also cause peripheral neuropathy that can lead to problems with numbness, tingling, or even pain in the hands and feet. Some patients develop shingles (herpes zoster) while taking this drug. To help prevent this, an anti-viral medicine (like acyclovir) may be prescribed.
Carfilzomib is a newer proteasome inhibitor that can be used to treat MM in patients who have already been treated with bortezomib and an immunomodulating agent. It is given as an injection into a vein, often in a four-week cycle. To prevent problems like allergic reactions during the infusion, the steroid drug dexamethasone is often given before each dose in the first cycle. Common side-effects include tiredness, nausea and vomiting, diarrhoea, shortness of breath, fever, and low blood counts. People on this drug can also have more serious problems such as pneumonia, heart problems, and kidney or liver failure.
Ixazomib is a proteasome inhibitor that is taken by mouth as a capsule, typically once a week for three weeks, followed by a week off. This drug is usually used after other drugs have been tried. Common side-effects of this drug include nausea and vomiting, diarrhoea, constipation, rash, swelling in the hands or feet, back pain, and a lowered blood platelet count. This drug can also cause peripheral neuropathy.
Histone deacetylase (HDAC) inhibitors
HDAC inhibitors are a group of drugs that can affect which genes are active inside cells. They do this by interacting with proteins in chromosomes called histones. Panobinostat (Farydak) is a HDAC inhibitor that can be used to treat patients who have already been treated with bortezomib and an immunomodulating agent. It is taken as a capsule, typically three times a week for two weeks, followed by a week off. This cyc e is then repeated. Common side-effects include diarrhoea (which can be severe), feeling tired, nausea, vomiting, loss of appetite, swelling in the arms or legs, fever, and weakness. This drug can also affect blood cell counts and the levels of certain minerals in the blood. Less common, but more serious, side-effects can include bleeding inside the body, liver damage, and changes in heart rhythm, which can sometimes be life-threatening.
Monoclonal antibodies
Daratumumab is a monoclonal antibody that attaches to the CD38 protein, which is found on myeloma cells. This is thought to both kill the cells directly and help the immune system attack the cells. This drug is used mainly in patients who have already had several other treatments for their myeloma. It is given as an infusion into a vein. This drug can cause a reaction in some people while it is being given or within a few hours afterward, which can sometimes be severe. Symptoms can include coughing, wheezing, trouble breathing, tightness in the throat, a runny or stuffy nose, feeling dizzy or lightheaded, headache, rash, and nausea. Other side-effects can include fatigue, nausea, back pain, fever, and cough. This drug can also lower blood cell counts, which can increase the risk of infections and bleeding or bruising.
Elotuzumab is a monoclonal antibody that attaches to the SLAMF7 protein, which is found on myeloma cells. This is thought to help the immune system attack the cells. This drug is used mainly in patients who have already had other treatments for their myeloma. It is given as an infusion into a vein. This drug can cause a reaction in some people while it is being given or within several hours afterward, which can sometimes be severe. Symptoms can include fever, chills, feeling dizzy or lightheaded, rash, wheezing, trouble breathing, tightness in the throat, or a runny or stuffy nose. Other common side-effects of this drug include fatigue, fever, loss of appetite, diarrhoea, constipation, cough, peripheral neuropathy, upper respiratory tract infections (such as a cold), and pneumonia.
Stem cell transplant for MM
In a stem cell transplant, the patient gets high-dose chemotherapy (sometimes with radiation to the whole body) to kill the cells in the bone marrow (including the myeloma cells). Then the patient receives new, healthy blood-forming stem cells. When stem cell transplants were first developed, the new stem cells came from bone marrow, and so this was known as a bone marrow transplant. Now, stem cells are more often gathered from the blood (a peripheral blood stem cell transplant).
Stem cell transplant is commonly used to treat MM. Before the transplant, drug treatment is used to reduce the number of myeloma cells in the patient’s body. Stem cell transplants are autologous
and allogeneic.
Autologous transplants
For an autologous stem cell transplant, the patient’s own stem cells are removed from his or her bone marrow or peripheral blood before the transplant. The cells are stored until they are needed for the transplant. Then, the person with myeloma gets treatment such as high-dose chemotherapy (eg, melphalan), sometimes with radiation, to kill the cancer cells. When this is complete, the stored stem cells are infused back into the patient’s blood. This type of transplant is a standard treatment for patients with MM. Still, while an autologous transplant can make the myeloma go away for a time (even years), it does not cure the cancer, and eventually the myeloma returns.
Some doctors recommend that patients with MM have two autologous transplants, six-to-12 months apart. This approach is called tandem transplant. Studies show that this may help some patients more than a single transplant. The drawback is that it causes more side-effects and so is riskier.
Allogeneic transplants
In an allogeneic stem cell transplant, the patient gets blood-forming stem cells from another person – the donor. The best treatment results occur when the donor’s cells are closely matched to the patient’s cell type and the donor is closely related to the patient such as a brother or sister. Allogeneic transplants are much riskier than autologous transplants, but they may be better at fighting the cancer. That is because transplanted (donor) cells may actually help destroy myeloma cells. This is called a graft vs tumor effect. Still, in studies of MM patients, those who got allogeneic transplants often did worse in the short-term than those who received autologous transplants. At this time, allogeneic transplants are not considered a standard treatment for myeloma, but may be done as a part of a clinical trial
The early side-effects from a stem cell transplant are similar to those from chemotherapy and radiation, only more severe. One of the most serious side-effects is low blood cell counts, which can lead to risks of serious infections and bleeding.
The most serious side-effect from allogeneic transplants is graft-versus-host disease (or GVHD). This occurs when the new immune cells (from the donor) see the patient’s tissues as foreign and so attack them. GVHD can affect any part of the body and can be life threatening.
Some patients are given additional cycles of treatment after transplant. This is called consolidation treatment and increases the chance of a complete response (where signs and symptoms of the disease go away).
Some patients (even some who did not have a stem cell transplant) may be given long-term treatment with thalidomide, lenalidomide, or bortezomib. This is known as maintenance treatment and helps delay the return of the myeloma, but it can cause serious side-effects.
Daratumumab in combination with bortezomib, thalidomide, and dexamethasone (VTd), has been granted reimbursement in Ireland for the treatment of patients with newly-diagnosed
MM who are eligible for autologous stem cell transplant.
The announcement followed European approval of daratumumab based on results from part one of the phase 3 CASSIOPEIA (MMY3006) study, published in The Lancet in June 2019 and presented at the 2019 American Society of Clinical Oncology (ASCO) Annual Meeting.
The Phase 3 CASSIOPEIA trial is a two-part study. Results from this first part of the trial showed that after consolidation, the stringent complete response (sCR) rate was significantly higher in the daratumumab-VTd arm (29 per cent) compared to VTd alone (20 per cent) (odds ratio [OR] = 1.60; 95 per cent confidence interval [CI], 1.21-2.12; P<0.0010). At a median follow-up of 18.8 months, progressive-free survival (PFS) was significantly improved in the daratumumab-VTd group compared to VTd alone (hazard ratio [HR] = 0.47; 95 per cent CI, 0.33-0.67; P<0.0001), and the median PFS was not reached in either arm. The addition of daratumumab to VTd resulted in an 18-month PFS rate of 93 per cent compared to 85 per cent for VTd alone.
The most common (≥10 per cent) grade 3/4 treatment-emergent adverse events (TEAEs) for daratumumab-VTd and VTd, respectively, were neutropaenia (28 per cent vs 15 per cent), lymphopaenia (17 per cent vs 10 per cent), stomatitis (13 per cent vs 16 per cent) and thrombocytopaenia (11 per cent vs 7 per cent). In the daratumumab-VTd combination arm, infusion-related reactions occurred in 35 per cent of patients.
CAR T-cell therapy
Chimeric antigen receptor (CAR) T-cell therapy, which is now available in Ireland, is a promising treatment for patients with MM whose disease has relapsed or is refractory to prior treatments. It is a highly-specialised therapy that involves genetically modifying a patient’s own T-cells to attack their MM using a target called B-cell maturation antigen (BCMA).
In the US, CAR T-cell therapy is approved for MM that has relapsed after, or is refractory to, at least four prior treatments. Clinical trials have showed CAR T-cell therapy to be highly effective for patients whose disease had relapsed after, or not responded to, multiple prior treatments.
Prognosis
MM is progressive and incurable, but median survival has recently improved to >five years as a result of advances in treatment. Unfavorable prognostic signs at diagnosis are lower serum albumin and higher beta-2 microglobulin levels. Patients initially presenting with renal failure also do poorly unless kidney function improves with therapy (which typically happens with current treatment options). Certain cytogenetic abnormalities increase risk of poor outcome.
Because MM is ultimately fatal, patients are likely to benefit from discussions of end-of-life care that involve their doctors and appropriate family and friends. Points for discussion may include advance directives, the use of feeding tubes, and pain relief.
References on request





Leave a Reply
You must be logged in to post a comment.